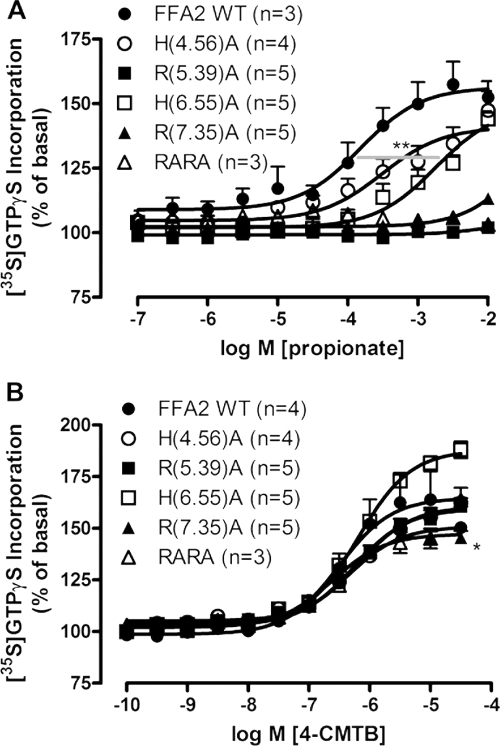
Fig. 3.
4-CMTB does not require a functional orthosteric ligand binding site for signaling. Flp-In TREx 293 cells stably expressing inducible wild-type or orthosteric binding site mutants of hFFA2-eYFP (Stoddart et al., 2008a) were induced with 0.5 μg/ml doxycycline for 24 h before harvesting and membrane preparation for subsequent [35S]GTPγS incorporation assays. A, mutation of either R(5.39)A, R(7.35)A, or both residues (RARA) completely abolishes propionate activity, whereas propionate potency is markedly impaired at the H(6.55)A mutation. **, p < 0.01 according to a one-way ANOVA with Dunnett's post hoc test. B, 4-CMTB potency is unaltered at any of the orthosteric binding site mutants of hFFA2-eYFP. Efficacy was only significantly impaired for R(7.35)A. *, p < 0.05 according to one-way ANOVA with Dunnett's post hoc analysis. Data are mean ± S.E.M with experiment repeats indicated in parentheses.