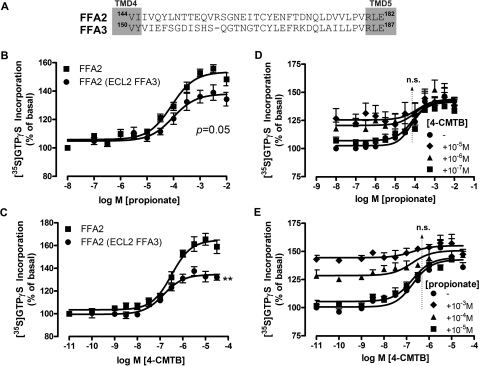
Fig. 8.
4-CMTB requires the second extracellular loop of hFFA2 for allosteric modulation of propionate. The 2nd extracellular loop of hFFA2 (Val144–Glu182) was replaced with ECL2 from the closely related hFFA3 receptor (Val150–Glu187) to generate an hFFA2(ECL2 FFA3)-eYFP chimeric protein and stable Flp-In TREx 293 cells generated as before. A, extracellular loop 2 alignment between hFFA2 and hFFA3 using ClustalX (accession numbers: hFFA2, NP005297; hFFA3, NP005295). B, propionate activates hFFA2(ECL2 FFA3)-eYFP with equivalent potency to hFFA2-eYFP WT (pEC50 = 3.96 ± 0.09 and 4.03 ± 0.14, respectively; n = 4) but with marginally reduced efficacy (p = 0.0501 according to an unpaired t test). C, 4-CMTB has significantly reduced efficacy at hFFA2(ECL2 FFA3) compared with hFFA2-eYFP WT (p < 0.001 according to an unpaired t test). Potency is equivalent between WT and chimeric receptors according to a t test (pEC50 = 6.50 ± 0.15 and 6.75 ± 0.11, respectively; n = 4). D, allosterism between 4-CMTB and propionate is lost at the hFFA2(ECL2 FFA3)-eYFP chimera. The arrow indicates potency; n.s. represents no significant difference in potency values according to one-way ANOVA (n = 3). E, reciprocal loss of allosterism at hFFA2(ECL2 FFA3)-eYFP according to one-way ANOVA and indicated by the arrow (n = 3).