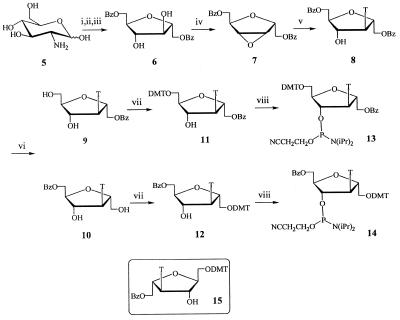
Figure 2.
Synthesis protocol of isonucleosides and isonucleoside phosphoramidites. Reagents and conditions: (i) NaNO2, concentrated HCl, H2O, 0°C; (ii) NaBH4, H2O, room temperature; (iii) BzCl, pyridine, CH2Cl2 (1:2 v/v), room temperature; (iv) PPh3, diethyl azodicarboxylate, 1,4-dioxane, 70°C; (v) thymine, DBU, DMF, 90–100°C; (vi) 5% NH3/CH3OH, room temperature; (vii) DMTrCl, pyridine, room temperature; (viii) chloro-(2-cyanoethoxy)-(diisopropylamine)phosphine, Et(i-Pr)2N, THF, room temperature. DMTr, 4,4′-dimethoxytrityl; Bz, benzoyl; T, thyminyl.