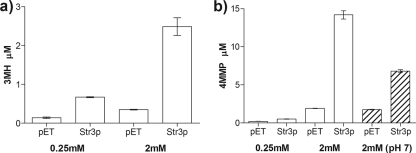
Fig. 3.
GC/MS quantification of enzymatic reactions with purified Str3p. The release of 3MH and 4MMP was quantified with headspace GC/MS in reaction mixtures incubated with 0.25 mM or 2 mM cysteine-S-conjugated precursor. Experiments were carried out with 31 μg/ml purified Str3p at 28°C and at pH 7.5 to minimize hydrolysis of the 4MMP precursor (26). Data shown are the means of triplicate experiments ± standard deviations of Str3p reactions and empty vector controls (pET), which were significantly different (P < 0.01) for both substrates. An additional negative control, using heat-inactivated Str3p, was indistinguishable from the empty vector control (P = 0.225 for 3MH and P = 0.442 for 4MMP). We observed a strong correlation (R2 = 0.95) between thiol and pyruvate formation for both Cys-3MH and Cys-4MMP (data not shown). Hatched bars show results of an experiment carried out at pH 7.0.