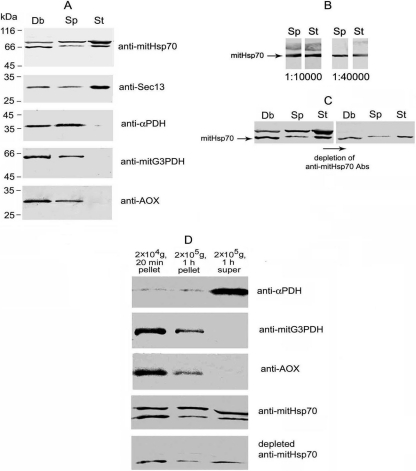
Fig. 1.
Western blot assay of A. locustae proteins. (A) Samples of spore homogenate cleared by centrifugation at 100 × g for 10 min (Sp) and stages of intracellular development (meronts and sporonts) ruptured by sonication (St) were equalized in protein concentration, and 20 μg was analyzed by immunoblotting. This experiment has clearly demonstrated specific accumulation of metabolic enzymes in microsporidial spores. To estimate protein diffusion from destroyed spores, an aliquot of crude debris (Db) resuspended in TS equal to the volume of homogenate was analyzed. (B) Double band recognized by anti-Hsp70 Abs represents two different proteins because serial dilution of anti-mitHsp70 immune serum up to 1:40,000 has resulted in drastic weakening of upper major band, whereas 66-kDa protein has remained specifically stained. (C) Specific decoration of 66-kDa protein by anti-mitHsp70 Abs depleted against major parasite protein of 75 kDa has confirmed the existence of unique epitopes in mitHsp70 polypeptide chain. Protein samples of Sp, St, and Db were prepared as described in the legend to panel A. (D) To analyze intrasporal localization of studied proteins, cleared spore homogenate was centrifuged in a series at 20,000 × g for 20 min and at 200,000 × g for 1 h, and the pellets were resuspended, with the volume of TS brought up to that of the final supernatant prior to Western blot assay. The pelleting of mitG3PDH, AOX, and most of mitHsp70 by high-speed centrifugation has suggested their localization in some membrane compartment.