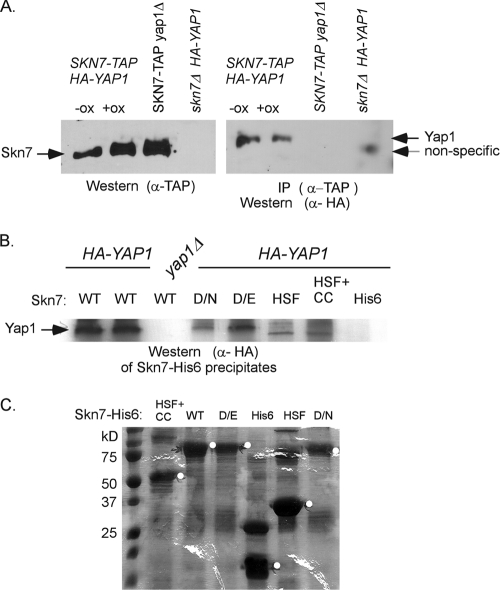
Fig. 3.
Coimmunoprecipitation of Yap1p depends on the Skn7p receiver domain. (A) Coimmunoprecipitation of HA-Yap1p in extracts expressing genomic SKN7-TAP. The presence of the TAP-tagged Skn7p in oxidant-treated and untreated extracts is shown in the α-TAP Western blot on the left. HA-Yap1p that immunoprecipitated with Skn7p-TAP is shown in the α-HA Western blot on the right. A nonspecific band is sometimes seen in extracts prepared from skn7Δ extracts. However, prolonged electrophoresis (shown) reveals that it migrates faster than HA-Yap1p. (B) Coprecipitation of HA-Yap1p with recombinant full-length Skn7p-His6 (WT), full-length Skn7p-D427N-His6 (D/N), full-length Skn7p-D427E-His6 (D/E), and truncated Skn7p-His6 constructs lacking the receiver domain but including the HSF DNA binding and the coiled-coil domains (HSF+CC) or including the HSF DNA-binding domain only (HSF). (C) SDS–10% PAGE and Coomassie blue staining of equivalent bead volumes (equal to 1/5 of the total) associated with each Skn7p-His6 protein. The remaining (4/5 total volume) beads were mixed with 0.5 mg of each yeast extract for the pull-down experiment shown in panel B except for wild-type beads, which were divided, with 2/5 being added to YAP1+ extract and 2/5 added to yap1Δ extract (first and third lanes in panel B). The white circles on the gel are to the right of bands migrating at the expected molecular mass of each Skn7p-His6 species.