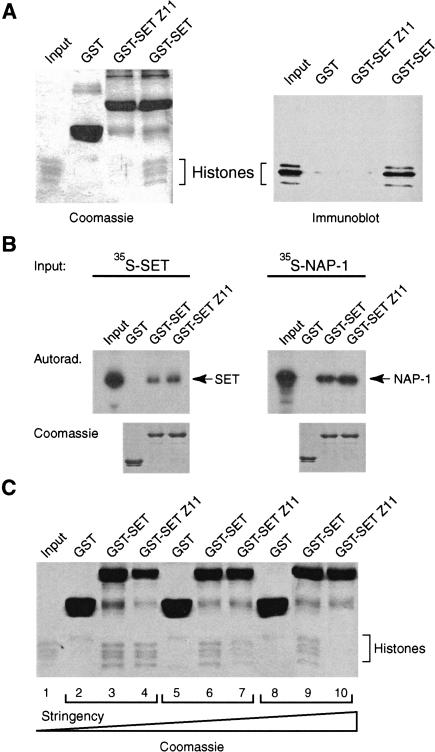
Figure 5.
The trxZ11 mutation impedes histone binding. (A) The histone-binding ability of equal amounts of GST–SET domain fusion protein containing the trxZ11 mutation GST–SET Z11 and wild-type GST–SET domain was compared in a pull-down experiment (see Fig. 1 legend ). Bound proteins were resolved by 15% SDS-PAGE and visualized by Coomassie staining or by Western immunoblotting using anti-histone antibodies. (B) The effect of the trxZ11 mutation on other protein–protein interactions was tested in a GST pull-down experiment. GST–SET domain or GST–SET Z11 fusion proteins were immobilized on glutathione–Sepharose and incubated with radiolabeled TRX SET domain or radiolabeled NAP-1 protein, respectively. Bound proteins were resolved by 15% SDS-PAGE and visualized by autoradiography (top panel). The bottom panels show the Coomassie stain of the same gels. (C) The histone binding experiment shown in A was repeated using variable binding conditions. (Lanes 2–4) 75 mM KCl, 0.02% NP-40; (lanes 5–7) 200 mM KCl, 0.02% NP-40; (lanes 8–10) 200 mM KCl, 0.08% NP-40. The increasing stringency of the binding conditions is indicated by a triangle (bottom). Final washes were with a buffer containing 600 mM NaCl and 0.2% NP-40.