Table 1.
Kinetic parameters for ht-ADH variants at pH 7, 30 °C
| Enzyme | kcat, s-1 | Km (Alcohol), mM | Km (NAD+), mM |
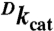 * *
|
| Wild type | 24.9 (±2.5) | 6.8 (±0.5) | 1.1 (±0.1) | 3.2 (±0.2) |
| L176V | 1.7 (±0.1) | 5.3 (±0.9) | 1.2 (±0.2) | 4.1 (±0.4) |
| L176A | 2.8 (±0.3) | 5.8 (±1.5) | 2.6 (±0.6) | 4.1 (±0.6) |
| L176G | 7.7 (±1.4) | 9.8 (±3.4) | 25 (±8) | 4.0 (±1.1) |
| L176Δ | 0.43 (±0.09) | 7.5 (±3.5) | 37 (±14) | 4.3 (±1.3) |
| V260A† | 2.4 (±0.2) | 4.2 (±0.8) | 10 (±1.6) | 4.1 (±0.7) |
*This is the ratio of kcat measured with H-benzyl alcohol to that measured with D-benzyl alcohol.
†The Arrhenius break for V260A is at approximately 40 °C; the rest are at 30 °C.
