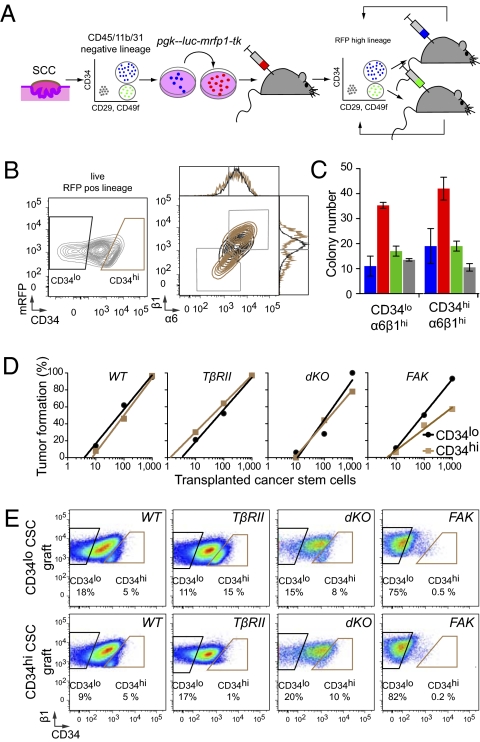
Fig. 2.
Cutaneous SCCs contain two interchangeable populations of CSCs that have tumor-initiating potential and are regulated by TβRII/TGF-β and FAK/integrin signaling. (A) Strategy to isolate CSCs. FACS-purified CD34hiCD29hiCD49fhiCD45−CD31−CD11b− cells are tagged with an RFP fluorescent reporter, and their long-term tumor-initiating potential is tested by serial orthotopic grafting experiments in immune-compromised Nude mice. (B) Representative flow cytometry profiles of RFP+ live cells isolated from secondary SCCs and separated based on their CD34 expression. Contour plots illustrate CD34lo (black) and CD34hi (brown) populations, which then were fractionated further based on α6(CD49f) and β1(CD29) integrin expression. Overlays illustrate that CD34hi subpopulations are either α6hiβ1hi or α6loβ1lo. (C and D) Colony formation (wild type, blue; TβRIIKO’ red; dKO,; green; FAKKO, gray; n > 2; error bars indicate SEM) and limit-dilution orthotopic transplantation experiments (n = 15–42 per data point; 576 grafts total) indicate that both α6β1hiCD34lo and α6β1hiCD34hi SCC cells form colonies in culture and tertiary SCCs in vivo, but their abilities depend upon TβRII and FAK function. (E) Flow cytometry profiles of tertiary SCCs illustrating the potential of CD34hi and CD34lo CSCs to generate CD34hi and CD34lo progeny interchangeably.