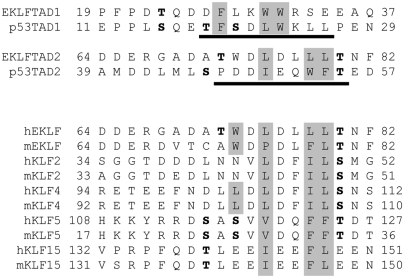
Fig. 1.
EKLFTAD1 and EKLFTAD2 are homologous to p53TAD1 and p53TAD2. (Top) Sequence alignments of EKLFTAD1 and EKLFTAD2 from human EKLF with p53TAD1 and p53TAD2 from human p53. The residues of p53 that form helices in the p53TAD1/MDM2 complex (23) and the p53TAD2/Tfb1PH complex (27) are underlined in black. The hydrophobic residues at the binding interfaces of the p53TAD1/MDM2 complex and the p53TAD2/Tfb1PH are in gray. Several known or potential phosphorylation sites are in bold. (Lower) Sequence alignment of regions of other KLF proteins (mouse and human) that share homology with EKLFTAD2. The KLF proteins are members of either KLF group 3 (EKLF, KLF2, KLF4) or KLF group 4 (KLF5, KLF15) based on phylogenetic analysis (1). Key hydrophobic residues are shaded gray and potential phosphorylation sites are in bold.