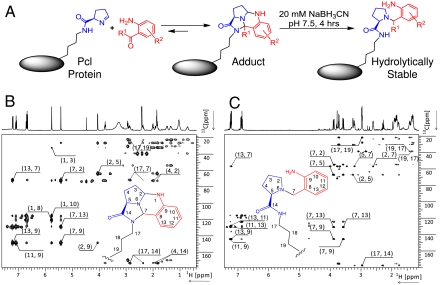
Fig. 4.
Site-specific modification of Pcl proteins through 2-ABA (R1 = H) and 2-AAP (R1 = CH3) derivatives. Examples of derivatives are shown in Table 1. (A) Structures of the protein adduct and the hydrolytically stable form after reduction as inferred from NMR studies with reacted Pcl amino acid. Summary of the key through bond connectivities detected by 1H-13C-HMBC NMR spectroscopy for the 2-ABA/Pcl amino acid adduct before (B) and after reduction (C). The first number of the crosspeak label identifies the 1H resonance while the second number identifies the 13C resonance of the respective atom or group. For numbering and structure, see inserts. Additional information, discussion, and NMR data for the 2-AAP adduct are provided as SI Appendix. Pcl was synthesized as a diastereomeric mixture (20). The stereochemistry of Pcl in the protein is drawn as observed for Pyl in proteins (26).