Abstract
Background
Nuclear factor κB (NFκB) plays a key role in the regulation of apoptosis. The function of NFκB is inhibited by binding to NFκB inhibitor (IκB), and disruption of the balance of NFκB and IκB is related to the development of many diseases, including tumors. Therefore, we hypothesized that the NFκB1 (-94del/insATTG) and NFκBIA (2758 A>G) polymorphisms were associated with colorectal cancer (CRC) susceptibility.
Methods
In a hospital-based case–control study of 1001 CRC patients and 1005 cancer-free controls frequency matched by age and sex, we genotyped polymorphisms using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method and performed luciferase assays and Western blotting analysis to identify whether genetic variants in NFκBIA alter its gene expressions and functions and thus cancer risk.
Results
We found that both NFκB1-94 ins/delATTG and NFκBIA 2758 A>G polymorphisms were correlated with CRC risk (OR = 1.47; 95%CI = 1.14–1.86, and OR = 1.38; 95% CI = 1.14–1.66, respectively). Furthermore, when evaluated these two polymorphisms together, the combined genotypes with 2 variant (risk) alleles (2758GG and -94ins/ins+del/ins) were associated with an increased risk of CRC (OR = 1.71; 95% CI = 1.23–2.38) compared to 0 variant, and the significant trend for 2 variant (risk) alleles were more pronounced among subgroups of aged <60 years, women, never drinkers, never smokers, persons with a normal BMI and those with a family history of cancer(Ptrend<0.01). Moreover, luciferase assays showed that the G allele in the 3′UTR significantly decreased NFκBIA mRNA stability and the A allele regulation by miRNA449a in vitro and that the NFκBIA protein expression levels of the AA+AG variant carriers were significantly higher in peritumoral tissues than those of the 2758GG genotype.
Conclusion
NFκB1 and NFκBIA polymorphisms appear to jointly contribute to risk of CRC. These two variants may be a genetic modifier for CRC susceptibility in this southern Chinese population.
Introduction
Colorectal cancer (CRC) is the third most common cancer in men and the second most common cancer in women around the globe, and it is estimated that there were approximately 1.2 million newly diagnosed CRC cases and 608,700 related deaths in 2008 [1]. Records from the municipal death registry of the city of Guangzhou, Guangdong, China, indicate that CRC is the fifth most common cancer. The mortality rate was dramatically increased from 4.33/105 persons in 1970's to 12.13/105 persons in 2000's [2]. The majority of CRC cases (approximately 80%) are sporadic [3], but a hereditary predisposition is present in 20–35% of patients, suggesting that both genetic and environmental factors contribute to CRC development [4]. Alcohol drinking and tobacco use [5], [6], dietary and lifestyle factors [7], and inflammatory bowel disease such as ulcerative colitis [8], [9] have shown to be associated with CRC risk. Although many people are exposed to these risk factors, only some of the exposed individuals develop CRC, indicating that genetic variation partly determines individual susceptibility to colorectal tumorigenesis.
Apoptosis, a highly regulated cellular process, participates in development, tissue homeostasis maintenance and elimination of unwanted cells [10]. Dysregulation in this process is likely to contribute to tumorigenesis [11]. The biochemical pathways of apoptosis are complicated and depend on not only the cells but also the inducers of apoptosis. Substantial evidence suggests that the occurrence and development of cancer is associated with both extended cell survival and suspended apoptosis in precancerous lesions and, consequently, aberrant apoptosis may allow for unchecked cell growth [12].
Nuclear factor kappa B (NFκB) is a major transcription regulator of the immune response, cell adhesion, differentiation, proliferation, and apoptosis [13]. Five members(p50/p105, p65/RelA, c-Rel, RelB, and p52/p100) in the NFκB family have been identified, and the dimeric form of NFκB1 p50/RelA is the major form [14]. In the resting cell, NFκB is inactivated in the cytoplasm through association with a sequestering inhibitory protein, IκBα, β or γ, and the most common protein of this family is the NFκB inhibitor α (NFκBIA) [15]. In the classical activation pathway, the phosphorylation and degradation of the inhibitory proteins lead to NFκB dissociation from the NFκB complex and translocation to the nucleus, where it can activate the transcription of a large number of genes [16]. As an important transcription factor, NFκB mediates the survival response by inhibiting p53-dependent apoptosis and up-regulating anti-apoptotic members of the Bcl-2 family and caspase inhibitors [17], [18]. In contrast, NFκB is also activated by both the extrinsic and intrinsic apoptotic stimuli and mediates upregulation of pro-apoptotic genes such as TRAIL R2/DR5, Fas, and Fas ligand [19], [20]. An inappropriate activation of NFκB could disturb tissue homeostasis and lead to dysregulated apoptosis. Furthermore, activity of NFκB has been observed in several types of cancers including CRC [21], [22], indicating it may play an important role in tumorigenesis [23], [24].
NFκB1 (encoding for NFκB) maps to chromosome 4q23–q24 and consists of 24 exons [25], [26], and its inhibitory gene NFκBIA (encoding for IκB) is 3.5 kb long, with six exons, and is located on chromosome 14q13 [27], [28]. Genetic studies have identified single nucleotide polymorphisms (SNP) in NFκB1 and NFκBIA [29], [30]. Recently, a common insertion/deletion (-94 insertion/deletionATTG rs28362491) polymorphism in the NFκB1 promoter region and a 3′ -untranslated region (3′UTR) polymorphism 2758A>G (rs696) in NFκBIA were observed to be significantly correlated with inflammatory bowel disease [31], [32] and cancers [33], [34], [35]. Epidemiological studies have also investigated the association between NFκB1 polymorphisms and risk of CRC in Germans and NFκBIA polymorphism and risk of CRC in the Swedish with conflicting results [36], [37].
There has been no previous report on the association between NFκB1 and NFκBIA polymorphisms and CRC risk. As the NFκB/IκB system plays an important regulatory role in the apoptotic pathway and dysregulated expression of the NFκB1 and NFκBIA has been observed in CRC, we hypothesized that combined NFκB1 and NFκBIA polymorphisms may be associated with increased risk of CRC. To test this hypothesis, we genotyped the NFκB1-94 insertion/deletionATTG and NFκBIA 2758A>G polymorphisms in our ongoing hospital-based case–control study of CRC in a southern Chinese population, and further performed luciferase assays and Western blotting analysis to identify whether genetic variants in NFκBIA alter its gene expressions and functions and thus cancer risk.
Materials and Methods
Ethics statement
The study protocol was approved by the institutional review boards of Sun Yat-Sen University. Written informed consent was obtained from each participant after a full explanation of the study.
Study subjects and sample collection
From July 2002 to April 2010, a total of 1001 patients with histopathologically-confirmed and untreated sporadic CRC were prospectively recruited from the First and Sixth Affiliated Hospital (Gastrointestinal & Anal Hospital) and Cancer Center of Sun Yat-Sen University, Guangzhou, China, the Affiliated Tumor Hospital of Guangzhou Medical College, Guangzhou, China, Guangdong Provincial People's Hospital, Guangzhou, China, and Panyu People's Hospital, Guangzhou, China. All these subjects were genetically unrelated ethnic Han Chinese and were from the city of Guangzhou and surrounding regions in southern China. Of the 1001 cases included in this study, there were 169 (16.9%) cases of right colon cancer, 309(30.9%)cases of left colon cancer, and 523 (52.2%) rectal cancer. According to American Joint Committee on Cancer staging Manual, there were 171 (17.1%)cases of stage I, 320 (31.9%)stage II, 345 (34.5%)stage III, and 165 (16.5%)stage IV. In the interim, a total of 1005 cancer-free controls were randomly selected from a subject pool of more than 10,000 individuals who participated in health check-up programs at the community health stations in Guangzhou, China.
The study participants were interviewed and data on smoking status, alcohol use and other factors including family history of cancer were obtained using a structured questionnaire. Smoking status, alcohol use and family history of cancer were defined as described previously [38]. Subjects whose body mass index (BMI) was <18.5 kg/m2 were categorized as being underweight, subjects whose BMI was from 18.5 to 24.0 kg/m2 were normal body weight, those who have a BMI >24.0 kg/m2 were overweight [39]. Cases belonging to familial adenomatous polyposis and those cases that fulfilled the criteria of Amsterdam for hereditary nonpolyposis CRC were excluded.
Genotyping
Five mL blood was collected from each participant and genomic DNA was extracted using the DNA Blood Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Genotyping was performed by the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. For determination of the NFκB1 promoter (rs28362491) polymorphism, the SNP-containing fragment was amplified using the following primers: 5′-TGGGCACAAGTCGTTTATGA-3′ (forward) and 5′-CTGGAGCCGGTAGGGAAG-3′ (reverse). PCR was run at 94°C for 3 min followed by 30 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 60 s with a final extension at 72°C for 10 min. The PCR products (281/285 bp in size) were digested with PfIMI (Fermentas, Vilnius, Lithuania) at 37°C overnight followed by 2% agarose gel electrophoresis. For determination of the NFκBIA (rs696) polymorphism, the SNP-containing fragment was amplified using the following primers: 5′-GGCTGAAAGAACATGGACTTG-3′ (forward) and 5′-GTACACCATTTACAGGAGGG -3′ (reverse). The PCR was run at 94°C for 5 min followed by 32 cycles of 94°C for 30 s, 54.3°C for 45 s and 72°C for 60 s with a final extension at 72°C for 10 min. The amplified fragments were digested with HaeIII (Fermentas, Vilnius, Lithuania) overnight at 37°C followed by 2% agarose gel electrophoresis.
Genotype analysis was done by two experimenters independently who were blinded to the status of the subjects as patient or control. To further validate the genotyping results, we randomly selected 10% samples for each of the 2 SNPs to perform repeat assays, and the results were 100% concordant. Additionally, 5% of the PCR products for each target genotype were purified and confirmed by direct sequencing (Figure S1 and Figure S2).
Bioinformatics analysis
To investigate whether genetic variants of NFκBIA could bind to microRNAs (miRNA), we searched for target microRNAs of genetic variants of NFκBIA using the algorithm programs (http://www.targetscan.org and http://microrna.sanger.ac.uk/cgi-bin/targets/v5/search.pl).
Cell culture
Three human colorectal adenocarcinoma cell lines, HCT116, HT29 and SW480 were purchased from Culture Collection of Chinese Academy of Science (Shanghai, China) and routinely cultured in Dulbecco's Modified Eagle's Medium or RPMI 1640 medium supplemented with 100 units/ml of penicillin, 100 µg/ml of streptomycin, and 10% fetal bovine serum (FBS). The cells were grown at 37°C with 5% CO2 in a humidified incubator.
RNA interference, transient transfections and luciferase assays
MiR-449a mimics targeting NFκBIA, 5′-UGGCAGUGUAUUGUUAGCUGGU-3′ (sense) and 5′-CAGCUAACAAUACACUGCCAUU-3′ (anti-sense), miR-449a inhibitor, 5′-ACCAGCUAACAAUACACUGCCA-3′, miR-34b mimics targeting NFκBIA, 5′-CAAUCACUAACUCCACUGCCAU-3′ (sense) and 5′-GGCAGUGGAGUUAGUGAUUGUU-3′ (anti-sense), and miR-34b inhibitor, 5′-AUGGCAGUGGAGUUAGUGAUUG-3′ were synthesized by GenePharma Co. (Shanghai, China). In addition, the 3′UTR of the NFκBIA 2758A allele (2575 to 2955 bp relative to the translation start site) containing the miR-449a and miR-34b binding site was amplified using the following two primers, 5′-CCGCTCGAGCAAAGGGGCTGAAAGAA-3′ (sense) and 5′-AAGGAAAAAAGCGGCCGCAAAATGTGGTCCTTCCATGA-3′ (anti-sense). The amplified fragments were restricted with XhoI and NotI (New England BioLabs, Ipswich, MA) and then ligated into the XhoI and NotI restriction site of the dual-luciferase plasmid, psiCHECK™-2 (Promega, Madison, WI). Additionally, the 3′-UTR of the NFκBIA 2758G allele, which contained a mutated mRNA binding site, was amplified using PCR site-directed mutagenesis and cloned into psiCHECK™-2. For transfection, cells were seeded onto 24-well plates at 1×105 cells/well and, after an overnight incubation, the cells were co-transfected with the reporter plasmid (2758G or 2758A allele ) and miR mimics or inhibitors using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Cells were lysed 48 h after transfection, and luciferase activity was measured using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Each experiment was done in triplicate and at least three times independently.
Western blotting analysis
To analyze the correlation between NFκBIA polymorphisms in its 3′UTR (2758A>G) and NFκBIA protein expression levels in tissues, 32 paired tumor and peritumoral tissues were obtained from sporadic CRC patients resected at the Sixth Affiliated Hospital of Sun Yat-Sen University and archived in the tumor bank. All tissues samples were histologically confirmed. Immunoblotting assays were performed as previously described [38] and antibodies against NFκBIA and β-actin (Precision Task Group, Chicago, USA) were used for the procedure. Protein densitometry was performed using Gel-Pro Analyzer 4.0 software (Media Cybernetics, Inc., Silver spring, MD) and NFκBIA expression was normalized against β-actin.
Statistical analysis
Two-sided chi-square tests were used to assess differences in the distributions of age, sex, smoking status, alcohol use, BMI, menstruation history, and family history of cancer between cases and controls. The Hardy-Weinberg equilibrium (HWE) was tested by a goodness-of-fit chi-square test to compare the expected genotype frequencies with observed genotype frequencies (p2+2pq+q2 = 1) in cancer-free controls. The association between case-control status and each SNP, measured by the odds ratio (OR) and its corresponding 95% confidence interval (CI), was estimated using an unconditional logistic regression model, with and without adjustment for age, sex, smoking status, alcohol drinking status, BMI, tumor site, and family history of cancer. Recent studies indicate that an analysis of the combined genotypes might be more scientifically significant than an analysis of a single polymorphism in predicting the disease associations. Therefore the combined genotype data were further stratified by age, sex, and smoking status, alcohol drinking status, BMI, tumor site, and family history of cancer. Logistic regression modeling was also used for the trend test. Student's t test was done to examine the difference in levels of luciferase reporter gene expression between different constructs. Kruskal-Wallis one-way ANOVA tests were used for analyzing NFκBIA protein expression in peritumoral tissues of different genotypes. All tests were two-sided by using the SAS software (version 9.1; SAS Institute, Cary, NC). P<0.05 was considered statistically significant.
Results
Characteristics of the study population
The distribution of demographic characteristics of the 1001 sporadic CRC cases and 1005 cancer-free controls are shown in Table 1 . Overall, no statistically significant difference was observed in age, sex, and smoking status between the cases and controls (P>0.05 in all). Compared with controls, there were more ever drinkers (cases vs. controls, 45.5% vs. 23.6%) (P<0.01) in the cohort of CRC cases. Moreover, compared with controls, CRC cases were more likely to have a family history of cancer or a higher BMI (P<0.05 in both). Consequently, these variables were further adjusted for in the multivariate logistic regression model to avoid possible confounding on the main effects of the SNPs under study. They were also used in the subsequent stratification and gene-environment interaction analysis.
Table 1. Frequency distributions of selected variables in CRC patients and cancer-free controls.
| Variables | 1001 Patients (n, %) | 1005 Controls (n, %) | P a | ||
| Age (years) | 0.4831 | ||||
| <50 | 233 | 23.3 | 231 | 23.0 | |
| 50–60 | 277 | 27.7 | 302 | 30.0 | |
| >60 | 491 | 49.0 | 472 | 47.0 | |
| Sex | 0.9487 | ||||
| Male | 604 | 60.3 | 605 | 60.2 | |
| Female | 397 | 39.7 | 400 | 39.8 | |
| Smoking status | 0.1964 | ||||
| Ever | 482 | 48.1 | 455 | 45.3 | |
| Never | 519 | 51.9 | 550 | 54.7 | |
| Alcohol drinking | <.0001 | ||||
| Ever | 456 | 45.5 | 237 | 23.6 | |
| Never | 545 | 54.5 | 768 | 76.4 | |
| Family history of cancer | 0.0122 | ||||
| Yes | 115 | 11.5 | 82 | 8.2 | |
| No | 886 | 88.5 | 923 | 91.8 | |
| BMI(kg/m2) | 0.0290 | ||||
| <18.5 | 63 | 6.3 | 39 | 3.9 | |
| 18.5–24.0 | 662 | 66.1 | 702 | 69.8 | |
| >24.0 | 276 | 27.6 | 264 | 26.3 | |
| Tumor site | |||||
| Right colon | 169 | 16.9 | |||
| Left colon | 309 | 30.9 | |||
| Rectum | 523 | 52.2 | |||
| Tumor Stages | |||||
| I | 171 | 17.1 | |||
| II | 320 | 31.9 | |||
| III | 345 | 34.5 | |||
| IV | 165 | 16.5 | |||
P values for a two-sided χ2 test.
PCR-RFLP study of the NFκB1 promoter region and the NFκBIA 3′UTR region
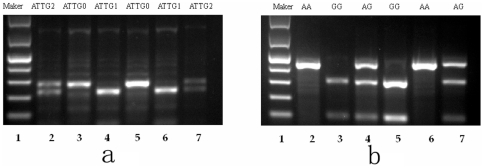
We carried out analysis of the NFκB1 promoter region by the PCR-RFLP method. Two ATTG repeats are present at the NFκB1 promoter region, and one allele that has an ATTG insertion (ins) was cleaved into a 45-bp and a 240-bp fragment after digestion with PfIMI while the other deletion allele (del) that has only one ATTG at its promoter was not cleaved (Figure 1.a). Furthermore, after digestion with HaeIII, the 2758GG genotype produced a 316-bp and a 108-bp band whereas the 2758AA genotype produced a single 424-bp band, and heterozygotes displayed all 3 bands (Figure 1.b).
Figure 1. Image of the cleavage products of the PfIMI and HaeIII restriction enzyme on 2% agarosegel.
(a) PfIMI digestion band profile of the NFκB1 gene on 2% agarosegel. Lane 1 Gene Ruler 100 bp Low Range Ladder, Lanes 2 and 7 del/ins heterozygote genotype, Lanes 4 and 6 ins/ins genotype, Lanes3, 5 del/del genotype. (b) HaeIII digestion band profile of NFκBIA on 2% agarosegel. Lane 1 Gene Ruler 100 bp Low Range Ladder, Lanes 3, 5 homozygote G/G genotype, Lanes 2 and 6 homozygote A/A genotype, Lanes 4, 7 heterozygote A/G genotype.
The NFκB1-94ins/delATTG (rs28362491) and NFκBIA 2758A>G (rs696) polymorphisms are associated with the risk of sporadic CRC
The genotype and allele distributions of the NFκB1-94ins/delATTG (rs28362491) and NFκBIA 2758A>G (rs696) polymorphisms among the cases and controls are summarized in Table 2. The observed genotype frequencies of these two polymorphisms were all in agreement with the Hardy–Weinberg equilibrium in the control subjects (P>0.05). As shown in Table 2 , for rs28362491, the distribution of the co-dominant genetic model (del/del vs. ins/ins vs. del/ins), and the dominant model (ins/ins+del/ins vs. del/del) differed significantly between CRC cases and controls (ins/ins: OR = 1.70; 95% CI = 1.29–2.25; P<0.01; del/ins: OR = 1.33; 95% CI = 1.03–1.74; P<0.01; ins/ins+del/ins: OR = 1.47; 95% CI = 1.14–1.86; P<0.01, respectively). For rs696, there was a significant difference in the distribution of the rs696 genotypes between CRC cases and controls (P<0.01). Significant differences were also noted in the distribution of the recessive model of rs696 (GG versus AA+AG) between CRC cases and controls (OR = 1.38; 95% CI = 1.14–1.66; P<0.05). Consistently, significant association was found between the two SNPs and the risk of sporadic CRC.
Table 2. Distribution of genotypes in NFκB1 and NFκBIA, and results of logistic regression analysis for associations with risk of colorectal cancer.
| Genotypes | Patients n (%) | Controlsa n (%) | P b | Crude OR (95% CI) | Adjusted OR (95% CI)c |
| Total no. of subjects | 1001 | 1005 | |||
| Total no. of alleles | 2002 | 2010 | |||
| NFκB1 Rs28362491 | 0.0008 | ||||
| del/del | 138 (13.8) | 186 (18.5) | 1.00 (ref.) | 1.00 (ref.) | |
| del/ins | 500 (49.9) | 522 (51.9) | 1.29 (1.01–1.66) | 1.33 (1.03–1.74) | |
| ins/ins | 363 (36.3) | 297 (29.6) | 1.65 (1.26–2.15) | 1.70 (1.29–2.25) | |
| 0.004 | |||||
| ins/ins+del/ins | 863 (86.2) | 819 (81.5) | 1.42 (1.12–1.81) | 1.47 (1.14–1.86) | |
| del/del | 138 (13.8) | 186 (18.5) | 1.00 (ref.) | 1.00 (ref.) | |
| NFκBIA Rs696 A>G | 0.0075 | ||||
| AA | 233 (23.3) | 212 (21.1) | 1.00 (ref.) | 1.00 (ref.) | |
| GA | 460 (45.9) | 531 (52.8) | 0.79 (0.63–0.99) | 0.78 (0.62–0.98) | |
| GG | 308 (30.8) | 262 (26.1) | 1.07 (0.83–1.37) | 1.04 (0.81–1.35) | |
| 0.0196 | |||||
| AA+GA | 693 (69.2) | 743 (73.9) | 1.00 (ref.) | 1.00 (ref.) | |
| GG | 308 (30.8) | 262 (26.1) | 1.38(1.15–1.65) | 1.38(1.14–1.66) | |
| Number of variant genotypesd | 0.0008 | ||||
| 0 | 100 (10.0) | 127 (12.6) | 1.00 (ref.) | 1.00 (ref.) | |
| 1 | 631 (63.0) | 675 (67.2) | 1.19 (0.89–1.58) | 1.22 (0.90–1.63) | |
| 2 | 270 (27.0) | 203 (20.2) | 1.69 (1.23–2.32) | 1.71 (1.23–2.38) | |
| Trend test P value | 0.0003 | 0.0004 |
The observed genotype frequencies among the control subjects were in agreement with the Hardy-Weinberg equilibrium (p 2+2pq+q 2 = 1) (χ2 = 3.539, P = 0.060 for Rs696G>A, P = 0.102 for Rs28362491 -94del/ins ATTG).
Two-sided χ2-test for the distribution of genotype frequency.
ORs were adjusted in a logistic regression model that included age, sex, smoking status, alcohol use, family history of cancer and BMI.
Either the Rs696GG or Rs28362491 ins/ins+del/ins genotypes are risk genotypes as one.
Stratification analysis of the association of combined NFκB1 and NFκBIA polymorphisms with risk of CRC
We further analyzed the combined genotypes of the NFκB1 and NFκBIA polymorphisms by examining age, sex, smoking, drinking, BMI, tumor site, and family history of cancer by logistic regression. We combined the NFκB1 and NFκBIA polymorphisms based on the number of variant (risk) alleles (i.e., 2758GG and -94ins/ins+del/ins). As shown in Table 3 , compared with the NFκB1-94 del/del and NFκB1IA 2758AA+AG genotype, one variant combined genotype carriers who were <60 years of age had a higher risk of CRC (OR = 1.57; 95% CI = 1.04–2.38) (P<0.05). Further stratification analysis revealed that individuals with two variants had a significantly higher risk of CRC among subgroups of patients aged <60 years (OR = 2.18; 95% CI = 1.37–3.44) (P<0.01), women (OR = 2.36; 95% CI = 1.35–4.10) (P<0.01), never smokers (OR = 2.05; 95% CI = 1.30–3.22) (P<0.01), never drinkers (OR = 1.87; 95% CI = 1.23–2.84) (P<0.01), persons with a normal BMI (OR = 1.80; 95% CI = 1.21–2.69) (P<0.01) and those with a family history of cancer (OR = 4.57; 95% CI = 1.34–15.6, P<0.05).
Table 3. Stratification analysis of the variant number of genotypes by selected variables in colorectal cancer patients and controls.
| Patients (n = 1001) | Controls (n = 1005) | Adjusted OR ( 95% CI)a | ||||||||
| Number of variant genotypesc | Number of variant genotypesc | |||||||||
| 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | P trend b | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||||
| Age (years) | ||||||||||
| ≤60 | 47 (9.2) | 328(64.3) | 135(26.5) | 78 (14.6) | 356(66.8) | 99(18.6) | 1.00 (ref.) | 1.57(1.04–2.38) | 2.18(1.37–3.44) | 0.0015 |
| >60 | 53 (10.8) | 303(61.7) | 135(27.5) | 49 (10.4) | 319(67.6) | 104(22.0) | 1.00 (ref.) | 0.89(0.56–1.41) | 1.28(0.77–2.11) | 0.0923 |
| Sex | ||||||||||
| Male | 67 (11.1) | 376 (62.2) | 161(26.7) | 81(13.4) | 393(65.0) | 131(21.6) | 1.00 (ref.) | 1.36 (0.90–2.05) | 1.49(0.98–2.27) | 0.0530 |
| Female | 33 (8.3) | 255(64.2) | 109(27.5) | 46(11.5) | 282(70.5) | 72(18.0) | 1.00 (ref.) | 1.30(0.80–2.11) | 2.36(1.35–4.10) | 0.0007 |
| Smoking status | ||||||||||
| Never | 46(8.9) | 329(63.4) | 144(27.7) | 71(12.9) | 370(67.3) | 109(19.8) | 1.00 (ref.) | 1.34(0.89–2.00) | 2.05(1.30–3.22) | 0.0006 |
| Ever | 51(11.2) | 302(62.7) | 126(26.1) | 56(12.3) | 305(67.0) | 9420.7) | 1.00 (ref.) | 1.09(0.68–1.73) | 1.42(0.87–2.33) | 0.0986 |
| Drinking status | ||||||||||
| Never | 53(9.7) | 338(62.0) | 154(28.3) | 94(12.2) | 950(69.0) | 144(18.8) | 1.00 (ref.) | 1.09(0.75–1.59) | 1.87(1.23–2.84) | 0.0002 |
| Ever | 47(10.3) | 293(64.3) | 116(25.4) | 33(13.9) | 145(61.2) | 59(24.9) | 1.00 (ref.) | 1.41(0.85–2.34) | 1.58(0.89–2.80) | 0.3055 |
| Family history of cancer | ||||||||||
| YES | 10(8.7) | 72(62.6) | 33(28.7) | 15(18.3) | 55(67.1) | 12(14.6) | 1.00 (ref.) | 2.50(0.91–6.87) | 4.57(1.34–15.6) | 0.0110 |
| NO | 90(10.2) | 559(63.1) | 237(26.7) | 112(12.1) | 620(67.2) | 191(20.7) | 1.00 (ref.) | 1.14(0.59–2.06) | 1.54(1.09–2.17) | 0.0048 |
| Tumor site | ||||||||||
| Right colon | 15(8.9) | 106(62.7) | 48(28.4) | 127(12.6) | 675(67.2) | 203(20.2) | 1.00 (ref.) | 1.18(0.82–1.69) | 1.59(1.07–2.37) | 0.0107 |
| Left colon | 32(10.4) | 193(62.4) | 84(27.2) | 127(12.6) | 675(67.2) | 203(20.2) | 1.00 (ref.) | 1.18(0.77–1.81) | 1.62(1.01–2.61) | 0.0238 |
| Rectum | 53(10.1) | 332(63.5) | 138(26.4) | 127(12.6) | 675(67.2) | 203(20.2) | 1.00 (ref.) | 1.42(0.79–2.55) | 1.94(1.02–3.67) | 0.0293 |
| BMI(kg/m2) | ||||||||||
| <18.5 | 11(17.5) | 38(60.3) | 14(22.2) | 3(7.69) | 28(71.8) | 8(20.5) | 1.00 (ref.) | 0.28(0.06–1.36) | 0.22(0.02–2.40) | 0.5447 |
| 18.5–24.0 | 65(9.8) | 414(62.5) | 183(27.7) | 91(13.0) | 470(66.9) | 141(20.1) | 1.00 (ref.) | 1.32(0.92–1.90) | 1.80(1.21–2.69) | 0.0022 |
| >24.0 | 24(8.7) | 179(64.9) | 73(26.4) | 33(12.5) | 177(67.1) | 54(20.4) | 1.00 (ref.) | 1.10(0.83–1.57) | 1.84(0.95–3.54) | 0.0644 |
ORs were adjusted for age, sex, smoking status, and alcohol use, BMI and family history of cancer in a logistic regression models.
Adjusted in a logistic regression model that included age, sex, smoking status, alcohol use, BMI and family history of cancer.
The number represents the numbers of variants within the combined genotypes, ie.0 = no variant (risk) allele and 1–2 = 1–2variant (risk) alleles; the variant (risk) alleles used for the calculation were the -94 ins/ins+del/ins and 2758GG alleles.
MiR-449a mimics suppressed the activities of the NFκBIA 2758 A>G polymorphism
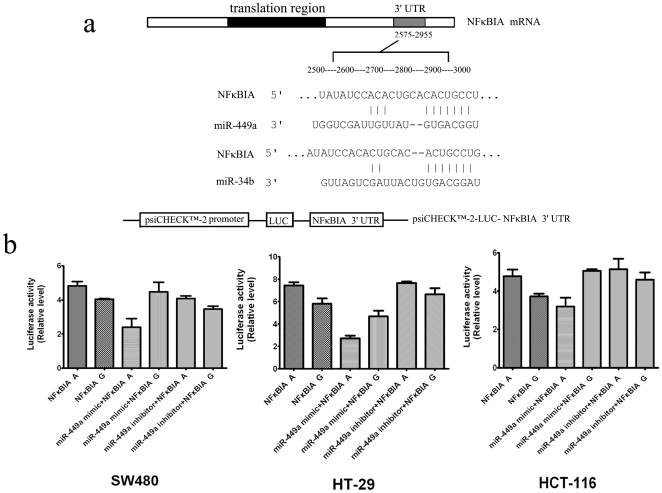
We further analyzed the 3′UTR of the NFκBIA 2758 A>G polymorphism using a computer algorithm and found that the polymorphism could affect miRNA binding. MiR-449a and miR-34b, which are involved in a wide variety of biological functions, were found to have a binding site within the 3′-UTR of the NFκBIA 2758 A>G polymorphism (Figure 2a). To determine the allele-specific effect of the NFκBIA 2758 A>G variant on the activity of the 3′UTR and whether this polymorphism affected miRNA binding, we transfected CRC cell lines SW480, HT29, and HCT116 with reporter plasmids carrying the 2758G or 2758A allele and miR mimics or inhibitors (Figure 2a). We found that, in the absence of miR mimics or inhibitors, compared with the 2758A allele, the 2758G allele exhibited decreased luciferase activities ( P<0.05)(Figure 2b). MiR-449a mimics could reduce the relative luciferase activities of the 2758A allele while miR-449a inhibitors significantly up-regulate the relative luciferase activities of the 2758A allele in all the three cell lines (P<0.01 for HT29; P<0.05 for SW480 and HCT116) (Figure 2b). On the other hand, miR-34b mimics failed to exert any noticeable effects on the relative luciferase activities of the 2758G allele in these cell lines (data not shown). Taken together, these results demonstrated that the 2758G allele in the 3′UTR of the NFκBIA reduced normalized luciferase activity compared to the 2758A allele, likely corresponding to reduced mRNA stability or translational efficacy and that miR-449a have the ability to bind and partially repress luciferase expression via the NFκBIA 3′UTR segment when carrying the 2758A allele of NFκBIA 2758 A>G polymorphism.
Figure 2. Validation of target microRNA predictions.
(a) A putative target site of miR-449a and miR-34b highly conserved in the NFκBIA mRNA 3′UTR. (b) psiCHECK-2 Dual Luciferase Assay in three cell lines: SW480, HT29 and HCT116. Cells were transfected with reporter plasmids alone or co-transfected with microRNA. Luciferase expression was measured 48 hrs after transfection. The luciferase activity of each construct was normalized against the internal control of Renilla luciferase. Columns, mean from three independent experiments; bars, SD. *, P<0.05 for all comparisons of each cell line between the activities of the reporter gene constructs.
Association of the NFκBIA3′UTR polymorphisms with the NFκBIA protein expression
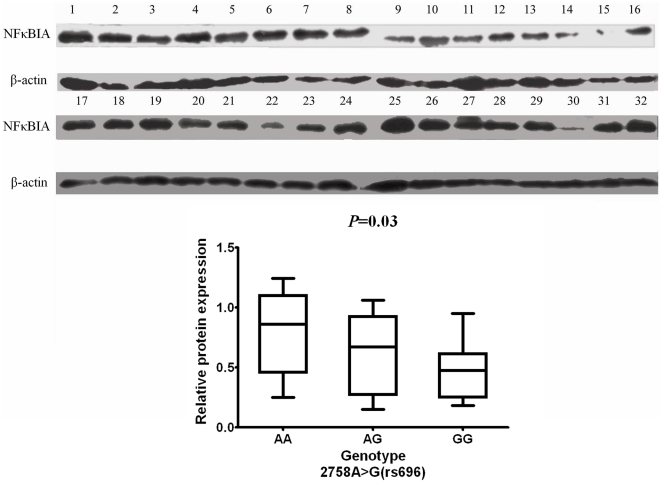
We were also interested in investigating whether the NFκBIA3′UTR polymorphisms (2758A>G) were associated with increased or reduced expression of NFκBIA protein. We collected 32 paired tumor and peritumoral tissues from the untreated CRC patients of different genotypes. Immunoblotting analysis revealed that the levels of NFκBIA (NFκBIA/β-actin protein ratio) in the peritumoral tissues of 12 cases of the 2758AA genotype was 0.80±0.09 and those of 8 cases of the 2758AG genotype was 0.63±0.11, both of which were significantly higher than those of the other 12 cases of the 2758GG genotype (0.47±0.06) (analysis of variance test, P<0.05) (Figure 3). However, in the tumor tissues, NFκBIA expression levels did not differ significantly among the cases of different genotypes (data not shown). These results indicated that NFκBIA levels were significantly higher in peritumoral tissues from patients of the 2758AA+AG genotypes than those of the 2758GG genotypes.
Figure 3. Association of the NFκBIA 3′UTR polymorphism with the NFκBIA protein expression.
NFκBIA protein levels in 32 sporadic CRC peritumoral tissues from individuals with different genotypes of 2758A>G. The NFκBIA protein expression levels were normalized to that of β-actin by calculating the relative expression levels. Individual genotype designation: For 2758A>G, lanes 1–3, 9–11, 17–20, 25–26, AA genotype (n = 12); lanes 4–6,12–14, 21–24, 27–28, GG genotype (n = 12); lanes 7–8, 15–16, 29–32, AG genotype (n = 8).
Discussion
In the present study, we investigated the associations of the NFκB1-94del/insATTG and NFκB1IA 2758A>G polymorphisms with risk of CRC in a southern Han Chinese population. We found that both the NFkB1-94del/ins ATTG and NFkBIA 2758A>G polymorphisms were associated with increased risk of CRC. For the NFkB1-94del/insATTG polymorphism, with the -94del/del as the reference, we found that the -94(ins/ins+del/ins) genotype was associated with a statistically significantly increased risk of CRC. For the NFKBIA 2758A>G polymorphism, with the 2758(AA+GA) as the reference, we also found that the 2758GG genotype was associated with a statistically significantly increased risk of CRC. Furthermore, when we evaluated NFkB1 and NFKBIA polymorphisms in combination, we found that the combined 2758GG and -94ins/ins+del/ins genotype was associated with a significantly increased risk of CRC compared with those without the 2758GG and -94ins/ins+del/ins genotype, and this increased risk was more pronounced among younger than 60 years, women, never drinkers, never smokers, persons with a normal BMI and those with family history of cancer. In the in vitro assays, we also found that, compared with the 2758A allele, the 2758G variant allele showed significantly decreased mRNA stability and/or translational efficacy. Furthermore, we found that NFkBIA levels significantly higher in peritumoral tissues from patients of the 2758AA+AG genotypes than those of the 2758GG genotypes, suggesting that the 2758A>G polymorphism is potentially functional and the polymorphism 2758A>G at 3′UTR of NFkBIA could affect gene expression. To the best of our knowledge, this is the first study to investigate whether NFkB1 and NFkBIA polymorphism and their combined polymorphism were associated with risk of CRC.
There are several lines of evidence supporting our findings. The NFκB1 pathways seem to play a critical role in multiple human pathologies by regulating the transcription of genes involved in the immune response, cell proliferation, and apoptosis [13]. It has been shown that alterations of NFκB1 expression plays an important role in the protection of cells from apoptosis [40]. NFκB1 activity has been observed in various types of cancer [41], including breast cancer [42] and CRC [21], to contribute to tumor angiogenesis and progression [43]. There are also many experimental data suggests that NFκB1/IκB pathway may participate in tumor cell invasion as well [44]. Therefore, the variants of the NFκB1 and NFκBIA genes, if functional, could be expected to have an effect on cell death, and thus, carcinogenesis.
Several association studies have reported that the NFκB1 and NFκBIA polymorphisms is related to the development of inflammatory and other diseases including ulcerative colitis, Graves' disease, and diabetes mellitus, and susceptibility to tumors including melanoma, bladder cancer and CRC in different ethnic groups [32], [36], [45], [46], [47], [48]. Gao et al.'s study reported a lack of an association between the NFκBIA2758 A>G polymorphism in northern Chinese population, but showed an association with the Swedish population and CRC risk [36]. Previous studies have provided evidence that the del allele may result in relatively decreased NFκB1 transcript levels and hence decreased p50/p105 NFκB1 protein production [32]. In our study, we further found that the change of the 2758 A to G allele in the 3′UTR of NFκBIA decreased luciferase activities as assessed by luciferase assays. Our functional in vitro experiments suggested that NFκBIA 2758 A>G variants may affect mRNA stability. However, that the NFκBIA 2758 A>G variants affects translational efficacy or conduces to differential nuclear RNA processing or export also cannot be completely excluded [49]. Furthermore, it is well known that miRNAs can also cause mRNA cleavage or translational repression by forming imperfect base pairing with the 3′-UTR of target genes. In silico analysis of the NFκBIA mRNA sequence predicted the 2758 A>G variant of NFκBIA generates a potential seed site for miR-449a and our ex vivo luciferase data indicated that miR-449a reduced the relative luciferase activities via the NFκBIA 3′UTR target site created by the 2758A allele. The results indicated that the 2758A allele strengthens the binding of miR-449a with 3′UTR of NFκBIA, which in turn inhibits the expression of NFκBIA. Recent evidence indicate that miRNAs can bind to the 3′ UTRs of mRNAs and affect their translation, thus regulating cell proliferation, apoptosis and tumorigenesis [50]. Experimental studies concluded that SNPs located in miRNA-binding sites affect miRNA target expression and function, which is potentially associated with cancers [51]. This reconcile with our results that the NFκBIA 2758A-allele may enhance binding of miR-449a and affect NFκBIA gene expression and change of the 2758 A to G allele in the 3′UTR of NFκBIA may affect mRNA stability or translational efficacy subsequent diminished protein levels. Consistently, we observed that NFκBIA protein expression was higher in the peritumoral tissues but not in tumor tissues from patients of the 2758AA+GG genotype than those of the 2758GG genotype. These data suggest that wildtype A allele may result in overexpression of NFκBIA in peritumoral normal tissues and hence increased NFκBIA protein production, resulting in inhibition of NFκB activity. However, carrying a mutant 2758G allele result in relatively decreased NFκBIA mRNA stability and hence diminished NFκBIA protein production. As a major inhibitor of NFκB, decreased expression and dysfunction of NFκBIA may be a direct result of the activation of NFκB and thus cancer [16]. This is also consistent with our initial expectations, since CRC has been associated with increased levels of NFκBIA G allele. These findings indicate that the NFκB1 -94del/ins ATTG and NFκBIA 2758 A>G polymorphisms are functional. Our data from this relatively large sample size study further support the notion that the NFκB1 and NFκBIA polymorphisms are potentially implicated in cancer risk.
In the present study, we also observed that the combined effect of the NFκB1 and NFκBIA polymorphisms on risk of CRC was more pronounced among younger than 60 years, women, never drinkers, never smokers, persons with a normal BMI and those with a family history of cancer, suggesting that, in these subpopulations, gene-environment interaction may be very weak and the combined effect was an independent risk factor for these subpopulations. Compared with the published data, our results indicate that the genotype distributions of the NFκB1 and NFκBIA polymorphisms vary with ethnicity. For example, the frequencies of the del/del, del/ins, and ins/ins genotypes of the NFκB1-94del/insATTG among our 1005 southern Han Chinese control subjects were 18.5%, 51.9%, and 29.6%, respectively, compared with 15.6%, 45.9%, and 38.4%, respectively, of 307 Germans in the study by Kathrin et al. [37]. Similarly, the frequencies of the AA, AG and GG genotypes of the NFκBIA 2758A>G in our controls were 21.2%, 52.8%, and 26.1%, respectively, compared with 6%, 45%, and 49%, respectively, of 109 cancer-free Australian controls in the study by Curran et al. [52]. However, the frequencies of the del/del, del/ins, and ins/ins genotypes of the NFκB1-94del/insATTG (18.5%, 51.9%, and 29.6%, respectively) were very similar to the published data by Sun et al. (17%, 58%, and 24%, respectively) [53]. In our southern Han Chinese controls, the frequency of the NFκBIA genotype distributions is also similar to that reported for that of the northern Han Chinese [36]. In this study, we also found that the insertion of NFκB1-94del/insATTG polymorphism increased CRC risk, which was contrary to the results reported by Andersen et al [54]. It is likely that the discrepancy results from the genetic difference in ethnicity (the frequencies of the del/del, del/ins, and ins/ins genotypes of the NFκB1-94del/insATTG among our 1005 southern Han Chinese control subjects were 18.5%, 51.9%, and 29.6%, respectively, compared with 13.5%, 45.9%, and 40.6%, respectively, of 756 Danes in the study). In addition, environmental effects such as dietary and lifestyle may also contribute to the discrepancy [7]. However, this hypothesis warrants further investigation.
In conclusion, our results suggested that both NFκB1 and NFκBIA polymorphisms have effect on risk of CRC. These findings suggest that the NFκB1 and NFκBIA polymorphisms may jointly contribute to the risk of CRC in a southern Chinese population, which were consistent with the functional assays we performed. Our study indicated that the NFκB1-94(ins/ins+del/ins) and NFκBIA GG polymorphism may be a genetic marker for susceptibility to CRC in Chinese populations. However, additional studies with more detailed data on environmental exposure and survival data are required to verify these findings. Therefore, future population-based studies are needed to verify the findings.
Supporting Information
NFκB1-94ins/delATTG genotyping by direct sequencing: (a) del/del genotype; (b) del/ins genotype; (c) ins/ins genotype.
(PPT)
NFκBIA 2758A>G genotyping by direct sequencing: (a) GG genotype; (b) AA genotype; (c) AG genotype.
(PPT)
Acknowledgments
The authors would like to thank all the participants for taking part in this research, especially Ms. Qinghua Huang, Peihuang Wu and Dr. Zhengyu Xian; Dechang Diao for their laboratory assistance; and gratefully acknowledge Defeng Chen for revising the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Natural Scientific Foundation of China grants [81072042 to Lei Wang, 81072366 to Jiachun Lu and 81001108 to Yisheng Wei], the Guangdong Provincial Scientific Research Grants [8151008901000059, 10251008901000008 to Lei Wang], the Gongdong province government, Education Department of Chinese Government, Science and Technology Department of Chinese Government [00590101220610034 to Jianping Wang], and the Research Fund for the Doctoral Program of Higher Education of China [200805580074 to Jianping Wang] and Yat-Sen Innovative Talents Cultivation Program for Excellent Tutors [88000-3126201 to Dianke Chen and Lei Wang] and supported by the Fundamental Research Funds for the Central Universities. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Cao KJ, Fan QY, Liu YL, Huang R, Yin CZ, et al. [Cancer incidence and mortality in Guangzhou City from 2000 to 2002]. Ai Zheng. 2008;27:225–230. [PubMed] [Google Scholar]
- 3.Cheah PY. Recent advances in colorectal cancer genetics and diagnostics. Crit Rev Oncol Hematol. 2009;69:45–55. doi: 10.1016/j.critrevonc.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, et al. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 5.Hoshiyama Y, Sekine T, Sasaba T. A case-control study of colorectal cancer and its relation to diet, cigarettes, and alcohol consumption in Saitama Prefecture, Japan. Tohoku J Exp Med. 1993;171:153–165. doi: 10.1620/tjem.171.153. [DOI] [PubMed] [Google Scholar]
- 6.Tsong WH, Koh WP, Yuan JM, Wang R, Sun CL, et al. Cigarettes and alcohol in relation to colorectal cancer: the Singapore Chinese Health Study. Br J Cancer. 2007;96:821–827. doi: 10.1038/sj.bjc.6603623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, et al. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171–180. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 8.von Roon AC, Reese G, Teare J, Constantinides V, Darzi AW, et al. The risk of cancer in patients with Crohn's disease. Dis Colon Rectum. 2007;50:839–855. doi: 10.1007/s10350-006-0848-z. [DOI] [PubMed] [Google Scholar]
- 9.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 12.Thompson HJ, Strange R, Schedin PJ. Apoptosis in the genesis and prevention of cancer. Cancer Epidemiol Biomarkers Prev. 1992;1:597–602. [PubMed] [Google Scholar]
- 13.Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blank V, Kourilsky P, Israel A. NF-kappa B and related proteins: Rel/dorsal homologies meet ankyrin-like repeats. Trends Biochem Sci. 1992;17:135–140. doi: 10.1016/0968-0004(92)90321-y. [DOI] [PubMed] [Google Scholar]
- 15.Hayden MS, West AP, Ghosh S. SnapShot: NF-kappaB signaling pathways. Cell. 2006;127:1286–1287. doi: 10.1016/j.cell.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore TD. The Re1/NF-kappa B/I kappa B signal transduction pathway and cancer. Cancer Treat Res. 2003;115:241–265. [PubMed] [Google Scholar]
- 17.Maldonado V, Melendez-Zajgla J, Ortega A. Modulation of NF-kappa B, and Bcl-2 in apoptosis induced by cisplatin in HeLa cells. Mutat Res. 1997;381:67–75. doi: 10.1016/s0027-5107(97)00150-4. [DOI] [PubMed] [Google Scholar]
- 18.Mayo MW, Wang CY, Cogswell PC, Rogers-Graham KS, Lowe SW, et al. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka H, Hoshikawa Y, Oh-hara T, Koike S, Naito M, et al. PRMT5, a novel TRAIL receptor-binding protein, inhibits TRAIL-induced apoptosis via nuclear factor-kappaB activation. Mol Cancer Res. 2009;7:557–569. doi: 10.1158/1541-7786.MCR-08-0197. [DOI] [PubMed] [Google Scholar]
- 20.Borset M, Hjorth-Hansen H, Johnsen AC, Seidel C, Waage A, et al. Apoptosis, proliferation and NF-kappaB activation induced by agonistic Fas antibodies in the human myeloma cell line OH-2: amplification of Fas-mediated apoptosis by tumor necrosis factor. Eur J Haematol. 1999;63:345–353. doi: 10.1111/j.1600-0609.1999.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 21.Yu HG, Yu LL, Yang Y, Luo HS, Yu JP, et al. Increased expression of RelA/nuclear factor-kappa B protein correlates with colorectal tumorigenesis. Oncology. 2003;65:37–45. doi: 10.1159/000071203. [DOI] [PubMed] [Google Scholar]
- 22.Lind DS, Hochwald SN, Malaty J, Rekkas S, Hebig P, et al. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery. 2001;130:363–369. doi: 10.1067/msy.2001.116672. [DOI] [PubMed] [Google Scholar]
- 23.Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- 24.Shibata W, Takaishi S, Muthupalani S, Pritchard DM, Whary MT, et al. Conditional deletion of IkappaB-kinase-beta accelerates helicobacter-dependent gastric apoptosis, proliferation, and preneoplasia. Gastroenterology. 2010;138:1022–1034 e1021–1010. doi: 10.1053/j.gastro.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heron E, Deloukas P, van Loon AP. The complete exon-intron structure of the 156-kb human gene NFKB1, which encodes the p105 and p50 proteins of transcription factors NF-kappa B and I kappa B-gamma: implications for NF-kappa B-mediated signal transduction. Genomics. 1995;30:493–505. doi: 10.1006/geno.1995.1270. [DOI] [PubMed] [Google Scholar]
- 26.Mathew S, Murty VV, Dalla-Favera R, Chaganti RS. Chromosomal localization of genes encoding the transcription factors, c-rel, NF-kappa Bp50, NF-kappa Bp65, and lyt-10 by fluorescence in situ hybridization. Oncogene. 1993;8:191–193. [PubMed] [Google Scholar]
- 27.Le Beau MM, Ito C, Cogswell P, Espinosa R, 3rd, Fernald AA, et al. Chromosomal localization of the genes encoding the p50/p105 subunits of NF-kappa B (NFKB2) and the I kappa B/MAD-3 (NFKBI) inhibitor of NF-kappa B to 4q24 and 14q13, respectively. Genomics. 1992;14:529–531. doi: 10.1016/s0888-7543(05)80261-7. [DOI] [PubMed] [Google Scholar]
- 28.Duerr RH, Barmada MM, Zhang L, Pfutzer R, Weeks DE. High-density genome scan in Crohn disease shows confirmed linkage to chromosome 14q11–12. Am J Hum Genet. 2000;66:1857–1862. doi: 10.1086/302947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ota N, Nakajima T, Shirai Y, Emi M. Isolation and radiation hybrid mapping of a highly polymorphic CA repeat sequence at the human nuclear factor kappa-beta subunit 1 (NFKB1) locus. J Hum Genet. 1999;44:129–130. doi: 10.1007/s100380050125. [DOI] [PubMed] [Google Scholar]
- 30.Glavac D, Ravnik-Glavac M, O'Brien SJ, Dean M. Polymorphisms in the 3′ untranslated region of the I kappa B/MAD-3 (NFKBI) gene located on chromosome 14. Hum Genet. 1994;93:694–696. doi: 10.1007/BF00201573. [DOI] [PubMed] [Google Scholar]
- 31.Klein W, Tromm A, Folwaczny C, Hagedorn M, Duerig N, et al. A polymorphism of the NFKBIA gene is associated with Crohn's disease patients lacking a predisposing allele of the CARD15 gene. Int J Colorectal Dis. 2004;19:153–156. doi: 10.1007/s00384-003-0531-y. [DOI] [PubMed] [Google Scholar]
- 32.Karban AS, Okazaki T, Panhuysen CI, Gallegos T, Potter JJ, et al. Functional annotation of a novel NFKB1 promoter polymorphism that increases risk for ulcerative colitis. Hum Mol Genet. 2004;13:35–45. doi: 10.1093/hmg/ddh008. [DOI] [PubMed] [Google Scholar]
- 33.Campbell KJ, Perkins ND. Regulation of NF-kappaB function. Biochem Soc Symp. 2006:165–180. doi: 10.1042/bss0730165. [DOI] [PubMed] [Google Scholar]
- 34.Zhou B, Rao L, Li Y, Gao L, Wang Y, et al. A functional insertion/deletion polymorphism in the promoter region of NFKB1 gene increases susceptibility for nasopharyngeal carcinoma. Cancer Lett. 2009;275:72–76. doi: 10.1016/j.canlet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Kim LH, Shin HD, Park BL, Jung JH, Kim JY, et al. Identification of variants in NFKBIA and association analysis with hepatocellular carcinoma risk among chronic HBV patients. Hum Mutat. 2003;21:652–653. doi: 10.1002/humu.9146. [DOI] [PubMed] [Google Scholar]
- 36.Gao J, Pfeifer D, He LJ, Qiao F, Zhang Z, et al. Association of NFKBIA polymorphism with colorectal cancer risk and prognosis in Swedish and Chinese populations. Scand J Gastroenterol. 2007;42:345–350. doi: 10.1080/00365520600880856. [DOI] [PubMed] [Google Scholar]
- 37.Riemann K, Becker L, Struwe H, Nuckel H, Duhrsen U, et al. No association of the NFKB1 insertion/deletion promoter polymorphism with survival in colorectal and renal cell carcinoma as well as disease progression in B-cell chronic lymphocytic leukemia. Pharmacogenet Genomics. 2006;16:783–788. doi: 10.1097/01.fpc.0000230414.74726.f6. [DOI] [PubMed] [Google Scholar]
- 38.Wei Y, Wang L, Lan P, Zhao H, Pan Z, et al. The association between −1304T>G polymorphism in the promoter of MKK4 gene and the risk of sporadic colorectal cancer in southern Chinese population. Int J Cancer. 2009;125:1876–1883. doi: 10.1002/ijc.24575. [DOI] [PubMed] [Google Scholar]
- 39.Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- 40.Sonenshein GE. Rel/NF-kappa B transcription factors and the control of apoptosis. Semin Cancer Biol. 1997;8:113–119. doi: 10.1006/scbi.1997.0062. [DOI] [PubMed] [Google Scholar]
- 41.Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biswas DK, Cruz AP, Gansberger E, Pardee AB. Epidermal growth factor-induced nuclear factor kappa B activation: A major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proc Natl Acad Sci U S A. 2000;97:8542–8547. doi: 10.1073/pnas.97.15.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 44.Amiri KI, Richmond A. Role of nuclear factor-kappa B in melanoma. Cancer Metastasis Rev. 2005;24:301–313. doi: 10.1007/s10555-005-1579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurylowicz A, Miskiewicz P, Bar-Andziak E, Nauman J, Bednarczuk T. Association of polymorphism in genes encoding kappaB inhibitors (IkappaB) with susceptibility to and phenotype of Graves' disease: a case-control study. Thyroid Res. 2009;2:10. doi: 10.1186/1756-6614-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller MR, Zhang W, Sibbel SP, Langefeld CD, Bowden DW, et al. Variant in the 3′ region of the IkappaBalpha gene associated with insulin resistance in Hispanic Americans: The IRAS Family Study. Obesity (Silver Spring) 2010;18:555–562. doi: 10.1038/oby.2009.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bu H, Rosdahl I, Sun XF, Zhang H. Importance of polymorphisms in NF-kappaB1 and NF-kappaBIalpha genes for melanoma risk, clinicopathological features and tumor progression in Swedish melanoma patients. J Cancer Res Clin Oncol. 2007;133:859–866. doi: 10.1007/s00432-007-0228-7. [DOI] [PubMed] [Google Scholar]
- 48.Riemann K, Becker L, Struwe H, Rubben H, Eisenhardt A, et al. Insertion/deletion polymorphism in the promoter of NFKB1 as a potential molecular marker for the risk of recurrence in superficial bladder cancer. Int J Clin Pharmacol Ther. 2007;45:423–430. doi: 10.5414/cpp45423. [DOI] [PubMed] [Google Scholar]
- 49.Conne B, Stutz A, Vassalli JD. The 3′ untranslated region of messenger RNA: A molecular ‘hotspot’ for pathology? Nat Med. 2000;6:637–641. doi: 10.1038/76211. [DOI] [PubMed] [Google Scholar]
- 50.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 51.Yu Z, Li Z, Jolicoeur N, Zhang L, Fortin Y, et al. Aberrant allele frequencies of the SNPs located in microRNA target sites are potentially associated with human cancers. Nucleic Acids Res. 2007;35:4535–4541. doi: 10.1093/nar/gkm480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curran JE, Weinstein SR, Griffiths LR. Polymorphic variants of NFKB1 and its inhibitory protein NFKBIA, and their involvement in sporadic breast cancer. Cancer Lett. 2002;188:103–107. doi: 10.1016/s0304-3835(02)00460-3. [DOI] [PubMed] [Google Scholar]
- 53.Sun XF, Zhang H. NFKB and NFKBI polymorphisms in relation to susceptibility of tumour and other diseases. Histol Histopathol. 2007;22:1387–1398. doi: 10.14670/HH-22.1387. [DOI] [PubMed] [Google Scholar]
- 54.Andersen V, Christensen J, Overvad K, Tjonneland A, Vogel U. Polymorphisms in NFkB, PXR, LXR and risk of colorectal cancer in a prospective study of Danes. BMC Cancer. 2010;10:484. doi: 10.1186/1471-2407-10-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NFκB1-94ins/delATTG genotyping by direct sequencing: (a) del/del genotype; (b) del/ins genotype; (c) ins/ins genotype.
(PPT)
NFκBIA 2758A>G genotyping by direct sequencing: (a) GG genotype; (b) AA genotype; (c) AG genotype.
(PPT)