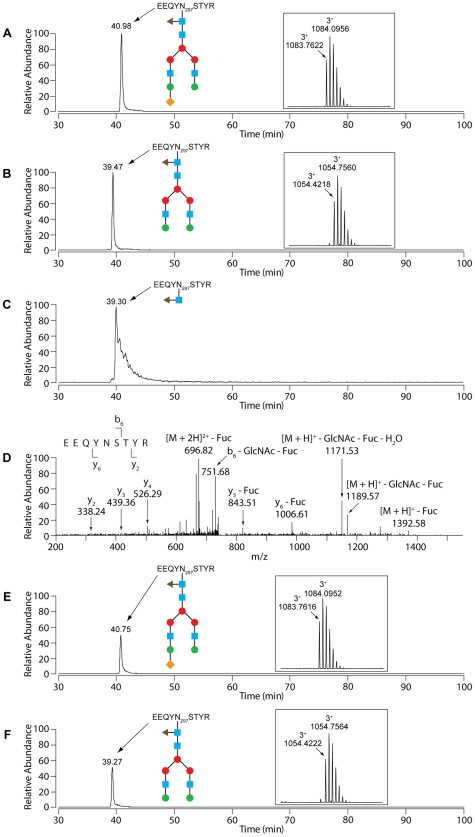
Figure 5. LC-MS analysis of IgG.
Glycosylation analysis of human IgG. (A) N297 glycosylation of untreated human IgG. Selected ion chromatogram for the triply charged tryptic peptide whose m/z is in accordance with a GlcNAc4Man3Fuc1Gal2Sial1 glycosyl moiety on the EEQYNSTYR peptide. The inset shows the isotope pattern for the N297 glycopeptide. The blue squares represent GlcNAc, the red and green circles Man and Gal, respectively, the orange diamonds Sial, and triangle Fuc. (B) N297 glycosylation of untreated human IgG. Selected ion chromatogram for the triply charged tryptic peptide whose m/z is in accordance with the GlcNAc4Man3Fuc1Gal2 glycosyl moiety on the EEQYNSTYR peptide. The inset shows the isotope pattern for the N297 glycopeptide. The sugar moieties are designated as in (A). (C) Selected ion chromatogram for the doubly charged N297 GlcNAc1Fuc1-modified glycopeptide of IgG that had been incubated with wild-type Cc5. The sugar moieties are designated as in (A). (D) Fragmentation spectrum of the N297 GlcNAc1Fuc1 species with the y- and b-ions that conclusively show the GlcNAc1Fuc1 modification of N297. (E) N297 glycosylation of human IgG that had been treated with the ΔgpdG strain: selected ion chromatogram for the triply charged tryptic peptide carrying the presumed GlcNAc4Man3Fuc1Gal2Sial1 glycosyl moiety on the EEQYNSTYR peptide. The inset shows the isotope pattern for the N297 glycopeptide. For the sugar structure see (A). (F) N297 glycosylation of human IgG that had been treated with the ΔgpdG strain: selected ion chromatogram for the triply charged tryptic peptide carrying the presumed GlcNAc4Man3Fuc1Gal2 glycosyl moiety on the EEQYNSTYR peptide. The inset shows the isotope pattern for the N297 glycopeptide. For the sugar structure see (A).