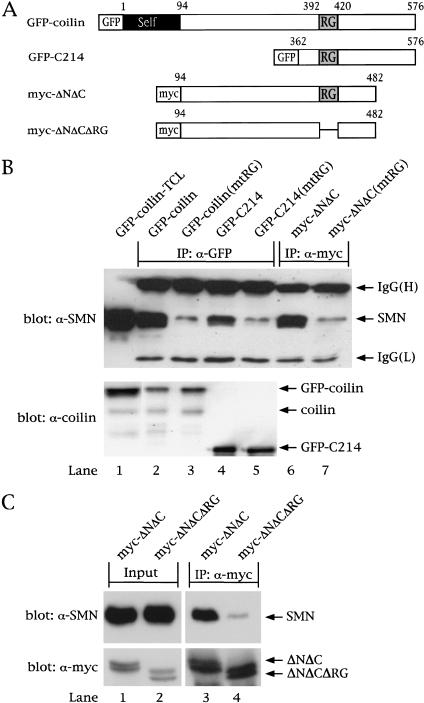
Figure 2.
The coilin RG box mediates interaction with SMN. (A) Schematic of coilin constructs. The RG box is indicated, as is the tag used for each construct. GFP–C214 contains the C-terminal 214 amino acids of coilin, residues 362–576. Myc-tagged coilinΔNΔC spans residues 94–482, as shown. The RG box of each construct was mutated (mtRG), as shown in Fig. 1A. Furthermore, the entire RG box (392–420) was deleted in myc–ΔNΔC (myc–ΔNΔCΔRG). (B) Coilin interaction with SMN is dependent on the RG box. HeLa cells were transfected with the various coilin constructs, and the lysates were immunoprecipitated with anti-GFP (lanes 2–5) or anti-myc (lanes 6,7) antibodies, as described in Materials and Methods. The beads were washed and subjected to SDS-PAGE and Western blotting with anti-SMN antibodies (top). The immunoglobulin (IgG) heavy (H) and light (L) chains are marked. The input lane shows 5% of the lysate used in the immunoprecipitation reactions. The same immunoprecipitates were electrophoresed and blotted with anticoilin antibodies to show that equal amounts of protein were pulled down (bottom). (C) Deletion of the RG box in coilin reduces SMN interaction. HeLa cells were transfected with myc–ΔNΔC or myc–ΔNΔCΔRG, and the lysates were immunoprecipitated with monoclonal anti-myc antibodies, followed by SDS-PAGE and Western blotting with anti-SMN antibodies (top). The same blot was reprobed with polyclonal anti-myc antibodies (bottom). The input lanes show 5% of the lysate used in the reactions.