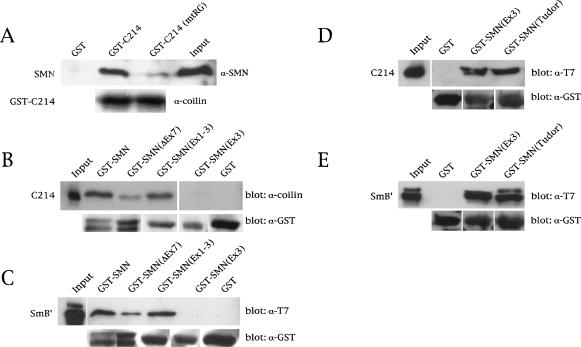
Figure 3.
Coilin directly interacts with SMN via the RG box. (A) Binding assays of His–SMN and GST–C214 or GST–C214(mtRG) were conducted as described in Materials and Methods, followed by SDS-PAGE and Western blotting with anti-SMN antibodies (top). The same blot was reprobed with anticoilin antibodies (bottom) to show that equal amounts of GST–C214 and GST–C214(mtRG) were used. (B) Mapping of the coilin interaction site on SMN. Various GST–SMN fusions were tested for their ability to bind His–C214. GST–SMNΔEx7 does not contain exon 7 sequences. GST–SMN(Ex1–3) and GST–SMN(Ex3) contain the first three or only the third exon of SMN, respectively. The Western blot was probed with an anticoilin antibody (top). The same blot was reprobed with anti-GST antibodies to verify that equal amounts of beads were used in the assay (bottom). (C) Control pulldowns with the same GST–SMN constructs used in B were assayed using His–T7–SmB′. Western blotting was performed with an anti-T7 antibody (top) or an anti-GST antibody (bottom). The input lanes for all reactions are equivalent to 10% of that used in the binding assay. (D,E) The Tudor domain of SMN mediates binding to coilin and SmB′. GST–SMN(Ex3), encompassing SMN residues 91–158, and GST–SMN(Tudor), spanning residues 83–173, were used in pulldown assays with purified coilin-C214 or SmB′. A less stringent buffer was used in these binding assays compared with the buffer used in A–C (see Materials and Methods). The blots were reprobed with an antibody to GST to verify that equal amounts of beads were used in the assay. The input lane for D was equivalent to 10% of the C214 used in the binding assay, whereas the input lane for E represents 20% of the SmB′ used in the binding reaction.