Abstract
Background
Anti-neutrophil cytoplasm antibody-associated vasculitis (AAV) can present with a broad spectrum of signs and symptoms. The relative effects of different manifestations on health related quality of life (HRQOL) is unknown.
Methods
We conducted an individual patient data meta-analysis of baseline Short Form 36 (SF-36) scores from four randomized controlled trials of patients with newly diagnosed AAV. We determined the associations between organ manifestations at trial entry and the SF-36 Physical Composite Score (PCS) and Mental Composite Score (MCS) using mixed effects models adjusted for demographic factors. Associations with each of the 8 domains of the SF-36 were further explored using multivariate multiple regression.
Results
SF-36 data was available from 346 patients. Older age (−0.11 points/year; 95% Confidence Interval [CI] −0.21 to −0.012; p=0.029) and neurologic involvement (−5.84, p<0.001) at baseline were associated with lower Physical Composite Scores. Physical Function scores were the most affected and older age (−0.25 points per year, 95% Confidence Interval [CI] −0.38 to −0.11; p<0.001) scores and neurologic involvement (−8.48 points, 95% CI −12.90 to −4.06; p<0.001) had the largest effects. The MCS was negatively affected only by chest involvement (p=0.027) but this effect was not exerted in any particular domain.
Conclusions
HRQOL in patients with newly diagnosed AAV are complex and incompletely explained by their organ system manifestations.
Keywords: vasculitis, health related quality of life, short form 36, ANCA, Wegener’s Granulomatosis
Introduction
Wegener’s granulomatosis (WG), microscopic polyangiitis (MPA) and renal-limited vasculitis are among the most common primary systemic vasculitides in adults. They are associated with circulating anti-neutrophil cytoplasm antibodies (ANCA) and, due to similarities in clinical features, histological characteristics, treatment and outcomes, are frequently grouped together as ANCA associated vasculitis (AAV). Earlier recognition of AAV and the widespread use of immunosuppressive treatment have significantly reduced its mortality (1;2). Patients with AAV are faced with a chronic medical condition and health related quality of life (HRQoL), the component of well being attributed directly to health status, is an increasingly important consideration.
Measuring HRQoL has been facilitated in the last 20 years by the development and validation of generic HRQoL instruments such as the Medical Outcomes Survey Short Form 36 (SF-36) (3;4). These instruments allow investigators to reliably measure several facets or domains of HRQoL in a multitude of conditions.
Despite the chronic morbidity observed in patients with AAV, there is little known about how disease manifestations affect HRQoL. Small, single centre studies examining what variables influence HRQoL have suggested that lung damage, joint involvement, and sino-nasal involvement are each been potentially important determinants of physical components of HRQoL in different studies (5–7). Determining which disease manifestations influence HRQoL and in what domains they affect HRQoL may help focus treatment for patients with AAV and help evaluate newer therapies. We studied the association between patient characteristics and particularly manifestations of AAV and HRQoL in a multi-centre cohort of patients that covered the spectrum of disease activity and manifestations.
Methods
Patients
The European Vasculitis Study Group conducted four trials that enrolled patients from 70 hospitals in 15 countries between 1995 and 2002 (8–11). All trials were conducted according to the 1964 Declaration of Helsinki and subsequent amendments. All patients were newly diagnosed with AAV (either Wegener’s granulomatosis, microscopic polyangiitis, or renal limited vasculitis). One trial enrolled patients with early systemic AAV (creatinine <150 μmol/L), two with generalized AAV (creatinine between 150 and 500 μmol/L), and one with severe AAV (creatinine >500 μmol/L or requiring dialysis). The individual trial eligibility criteria are summarized in Table 1.
Table 1.
Summary of included trial eligibility and treatment regimens.
| Trial | Included Disease Stage | Included Creatinine (μmol/L) | Induction Treatment | Maintenance Treatment |
|---|---|---|---|---|
| NORAM | Early Systemic | <150 | MTX vs. oral CYC | MTX vs. oral CYC |
| CYCAZAREM | Generalized | 150 – 499 | oral CYC | Oral CYC vs. AZA |
| CYCLOPS | Generalized | 150 – 499 | IV CYC vs. oral CYC | AZA |
| MEPEX | Severe | ≥ 500 | PE+ oral CYC vs. IVMeP + oral CYC | AZA |
MTX = methotrexate; CYC = cyclophosphamide; AZA = azathioprine; IV = intravenous; PE = plasma exchange; IVMeP = intravenous methylprednisolone
Measures
HRQoL was evaluated with the Short-Form 36 Health Survey, a generic self-reported health questionnaire administered in the patient’s native language whenever possible. The SF-36 measures HRQoL in eight domains, four physical (Physical Function, Role Physical, Bodily Pain, and General Health) and four mental (Social Functioning, Role Emotional, Mental Health, and Vitality). The score for each domain was normalized to United Kingdom population scores with a mean of 50 and standard deviation of 10 with higher scores indicating better quality of life (12;13). In addition, domains are summarized as a Physical Composite Score (PCS) and Mental Composite Score (MCS) also with a population mean of 50 and standard deviation of 10. A 5 point difference in scores is generally regarded as the minimum clinically important difference (MCID) (14).
Patients were assessed at baseline for manifestations of AAV in each organ system using the Birmingham Vasculitis Activity Score (BVAS), an instrument with 9 domains (general, cutaneous, mucous membranes/eyes, ears/nose/thoat [ENT], chest, cardiovascular, abdominal, renal, and nervous system) (15). Each BVAS item is scored if the sign or symptom started or worsened over the four weeks prior to the evaluation. The BVAS produces a summary score for overall disease activity that can range from 0 to 63. The summary score is composed of the sum of each organ domain specific scores. For the purpose of this analysis, each organ domain was classified as actively involved or not on the basis of ≥1 item or no items present at baseline. Serum creatinine was measured at baseline and converted to an estimated glomerular filtration rate (eGFR) using the four variable MDRD equation (16). AAV was sub-grouped as Wegener’s granulomatosis or microscopic polyangiitis (including renal limited vasculitis) according to the Chapel Hill Consensus Statement (17).
Statistical Analyses
Summary data is presented as mean (SD) or median (interquartile range [IQR]) as appropriate for normal and non-normally distributed continuous variables respectively. Baseline characteristics between those included and those excluded for analysis were compared by student’s t-test for normally distributed continuous variables, Mann-Whitney test for non-normally distributed continuous variables and Fisher’s exact test for dichotomous data.
Associations between baseline characteristics and PCS and MCS scores were determined using mixed-effects linear regression in which each trial served as a random effect. Identical models were fit for PCS and MCS data. Each model included age, sex, eGFR, diagnosis (WG vs MPA or renal-limited vasculitis) and organ system involvement for each of the nine BVAS organ systems. To explore whether each baseline variable was associated with certain physical or mental domains, we assessed all physical domains simultaneously in a multivariate regression model and all mental domains in a second model. Predictor variables in the multivariate regression models were specified in the same way as the multilevel models but without a random effect for trial. Models for Physical Function, Role Physical, Bodily Pain and General Health scores were simultaneously fit for physical domains and models for Social Function, Mental Health, Role Emotional and Vitality scores were simultaneously fit for mental domains model. Missing predictor covariate data was imputed using chained equation multiple imputation techniques (18;19). Ten imputation datasets were used to generate all final analyses. Sensitivity analyses using only complete cases were also conducted. Sensitivity analyses in which pulmonary hemorrhage (defined as hemoptysis or respiratory failure attributed to active vasculitis) where coded separate from other chest manifestations were also conducted to ensure that estimates for chest involvement were not driven solely by pulmonary hemorrhage. Further sensitivity analyses that included the summary BVAS as a measure of overall disease activity and excluded individual organ involvement variables were conducted. A p-value < 0.05 was considered statistically significant with no corrections for multiple comparisons for the PCS and MCS models. In multivariate models, type I errors due to multiple comparisons were contained by adjusting the significance level by the number of covariates in the model (i.e. adjusted p<0.004 for significance) in the multivariate models. A point estimate of at least 5 points was required to be considered clinically significant. All analyses were performed on Stata v11 (College Station, TX).
Results
Patients
A total of 535 patients were enrolled in the four trials. Of these, 346 (65%) had baseline SF-36 data for analysis. Eighty-four percent of NORAM patients, 72% of CYCAZAREM patients, 51% of CYCLOPS patients and 57% of MEPEX patients completed baseline SF-36 evaluations. Patients with SF-36 data more frequently had WG and ENT manifestations, had better renal function, lower BVAS, and less frequently had general, or renal manifestations compared to those who did not have SF-36 data (Table 2). Eighty-four percent of patients with SF-36 data had complete covariate data available for analysis; in the remaining 16%, at least one predictor variable was multiply imputed.
Table 2.
Characteristics of patients included and excluded from this study.
| Included N=346 |
Excluded N=189 |
p-value† | |
|---|---|---|---|
| Mean Age (SD), years | 57.1 (13.9) | 58.4 (14.9) | 0.39 |
| Female (%) | 43.9% | 50.2% | 0.15 |
| Wegener’s Granulomatosis (%) | 58.5% | 41.6% | <0.001 |
| Mean Baseline BVAS (SD) | 17.6 (8.5) | 19.2 (8.4) | 0.041 |
| Median eGFR (IQR), ml/min | 33.5 (10.9 to 70.0) | 18.9 (7.6 to 51.9) | <0.001‡ |
| Organ Involvement (%) | |||
| General | 91.9 | 87.1 | 0.009 |
| Cutaneous | 23.5 | 23.9 | 0.91 |
| Mucous Membrane/Eye | 30.4 | 26.4 | 0.22 |
| ENT | 52.9 | 46.0 | 0.036 |
| Chest | 52.5 | 46.6 | 0.071 |
| Cardiac | 5.7 | 4.9 | 0.69 |
| Abdominal | 4.7 | 6.7 | 0.18 |
| Renal | 86.8 | 92.3 | 0.007 |
| Neurologic | 20.1 | 20.8 | 0.81 |
p-values from t-tests for continuous variables or Fisher’s exact test for categorical variables except where noted;
p-value from Mann-Whitney test.
SD = standard deviation; BVAS = Birmingham Vasculitis Activity Score; eGFR = estimated glomerular filtration rate; IQR = interquartile range; ENT = ears, nose and throat.
Distribution of SF-36 Scores
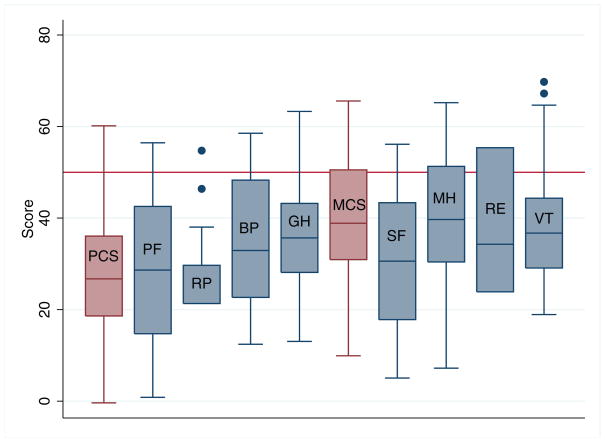
Figure 1 demonstrates the distribution of SF-36 scores for all patients. For PCS, the mean (standard deviation) was 27.6 (12.5) and the median (interquartile range [IQR]) was 26.7 (18.6 to 36.1). The MCS mean was 40.4 (11.9) and the median was 38.9 (30.9 to 50.5). Both the PCS and MCS were significantly lower than the population norm of 50 (p<0.001 for both PCS and MCS compared to population norms). Of the physical domains, Physical Function and Role Physical scores were the lowest with medians (IQR) of 28.6 (14.7 to 42.5) and 21.3 (21.3 to 29.7) respectively. Amongst the mental domains, Social Function scores were the lowest with a median (IQR) of 30.6 (17.8 to 43.4).
Figure 1.
Distribution of Short Form 36 scores in patients with ANCA associated vasculitis. Population average is 50 (horizontal line). Boxes represent 25th to 75th percentile with median (embedded horizontal line). Whiskers represent 5th to 95th percentile and dots represent outliers. PCS = Physical Composite Score; PF = Physical Function; RP = Role Physical; BP = Bodily Pain; GH = General Health; MCS = Mental Composite Score; SF = Social Functioning; MH = Mental Health; RE = Role Emotional; VT = Vitality.
Associations with Physical and Mental Composite Scores
Older age was independently associated with lower PCS (p=0.029) although a 45 year age difference was required to reach the minimum clinically important difference (0.11 points per year of age). Neurologic activity was the only organ system independently associated with a statistically (p<0.001) and clinically significant (−5.84 points; 95% CI −2.60 to −9.09 points) reduction in PCS (Table 3). Chest involvement was associated with a statistically (p=0.027) but not clinically significant (−2.96 points; 95% CI −0.33 to −5.58) reduction in MCS scores (Table 3). No other factors were associated with a significant reduction in MCS scores. Sensitivity analyses using only complete cases did not differ materially from analyses utilizing multiple imputations. Similarly, sensitivity analyses in which pulmonary hemorrhage was considered separately from other chest manifestations did not differ materially from primary analyses and estimates for the effect of pulmonary hemorrhage were similar to the estimates for other chest manifestations.
Table 3.
Mixed-effects multivariable regression models for Physical Composite and Mental Composite Scores of the Short Form 36 questionnaire.
| PCS | MCS | |||
|---|---|---|---|---|
| β (95% CI) | p-value | β (95% CI) | p-value | |
| Age (per year) | −0.11 (−0.21 to −0.012) | 0.029 | 0.036 (−0.066 to 0.14) | 0.49 |
| Sex | −2.38 (−4.98 to 0.21) | 0.072 | −2.32 (−4.88 to 0.24) | 0.076 |
| Diagnosis (MPA) | 0.68 (−2.85 to 4.22) | 0.71 | 2.34 (−1.19 to 5.87) | 0.19 |
| eGFR (per 10 ml/min) | 0.058 (−0.48 to 0.59) | 0.83 | 0.38 (−0.11 to 0.87) | 0.13 |
| Organ Involvement | ||||
| Systemic | −4.83 (−11.08 to 1.41) | 0.13 | −4.98 (−11.23 to 1.26) | 0.12 |
| Cutaneous | −2.42 (−5.51 to 0.66) | 0.12 | 2.22 (−0.87 to 5.32) | 0.16 |
| Mucous Membrane/Eye | −2.50 (−5.44 to 0.45) | 0.096 | −0.48 (−3.45 to 2.48) | 0.75 |
| ENT | −1.79 (−5.04 to 1.46) | 0.28 | 2.97 (−0.27 to 6.22) | 0.072 |
| Chest | −2.26 (−4.86 to 0.35) | 0.089 | −2.96 (−5.58 to −0.33) | 0.027 |
| Cardiac | 0.82 (−5.20 to 6.84) | 0.79 | −0.97 (−7.08 to 5.12) | 0.75 |
| Abdominal | 1.69 (−4.86 to 8.25) | 0.61 | 5.20 (−1.45 to 11.84) | 0.13 |
| Renal | −2.85 (−7.18 to 1.48) | 0.20 | 0.63 (−3.21 to 4.48) | 0.75 |
| Neurologic | −5.84 (−9.09 to −2.60) | <0.001 | 0.076 (−3.22 to 3.37) | 0.96 |
Although few individual organ systems are associated with clinically and statistically significant differences in the PCS and MCS, their combined effects may result in clinically significant differences particularly considering most patients have several organ systems involved at the time of diagnosis (median 4 systems). The estimated reduction in PCS for an individual with the four most common organ manifestations (systemic, renal, ENT and chest) of 22 points (when considering age) is similar to the observed 22.6 point reduction in PCS in our study compared to the normal population. Sensitivity analyses that included BVAS score as an overall measure of disease severity did not show any independent association between BVAS and either PCS or MCS. Thus, for most patients with newly diagnosed AAV, overall HRQoL may be largely a function of having active disease rather than a function of activity in particular organs or severity of activity.
Associations with Individual Domain Scores
The results of multivariate regression to explore the association of baseline characteristics with each domain of the SF-36 are summarized in Table 4 (physical domains) and Table 5 (mental domains). A p-value of <0.004 was required for statistical significance in the joint multivariate models to reduce the type I error rate. Older age was associated with lower Physical Functioning (p<0.001). An age difference of 20 years was required to reach the MCID of 5 points. Female sex and renal function demonstrated trends towards effects in several domains but none of these met our significance threshold. Furthermore, the point estimates for the effect of female sex did not meet the MCID and for renal function, the difference in eGFR required to meet the MCID was approximated 80 ml/min (i.e. the MCID was only met if comparing patients requiring dialysis to those with near normal renal function). There was no difference between those patients with WG and those with MPA in any domain of the SF-36.
Table 4.
Multivariate model of association of patient characteristics with physical domains of the Short Form 36 questionnaire.
| Physical Functioning | Role Physical | Bodily Pain | General Health | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | |
| Age | −0.25 (−0.38 to −0.11) | <0.001 | −0.10 (−0.19 to −0.01) | 0.027 | 0.061 (−0.07 to 0.19) | 0.36 | −0.42 (−0.13 to 0.045) | 0.34 |
| Female | −4.46 (−7.84 to −1.08) | 0.010 | −1.42 (−3.71 to 0.87) | 0.22 | 0.008 (−3.27 to 3.29) | 0.99 | −2.03 (−4.21 to 0.16) | 0.069 |
| MPA | 0.61 (−4.03 to 5.26) | 0.80 | 1.61 (−1.52 to 4.75) | 0.31 | −0.20 (−4.70 to 4.29) | 0.93 | 2.15 (−0.84 to 5.14) | 0.16 |
| eGFR (per 10 ml/min) | 0.64 (0.0024 to 1.29) | 0.049 | 0.17 (−0.26 to 0.61) | 0.43 | −0.04 (−0.67 to 0.58) | 0.90 | 0.25 (−0.18 to 0.67) | 0.25 |
| Organ Involvement | ||||||||
| General | −5.32 (−4.72 to 3.66) | 0.21 | −4.90 (−10.47 to 0.68) | 0.085 | −3.48 (−11.49 to 4.54) | 0.39 | −6.50 (−12.07 to −0.93) | 0.022 |
| Cutaneous | −0.53 (−4.72 to 3.66) | 0.80 | −0.35 (−3.10 to 2.40) | 0.80 | −3.02 (−7.03 to 0.99) | 0.14 | 0.78 (−1.99 to 3.53) | 0.58 |
| Mucous Membrane/Eye | −2.40 (−6.37 to 1.56) | 0.23 | −0.42 (−3.05 to 2.21) | 0.76 | −3.55 (−7.35 to 0.25) | 0.067 | −0.12 (−2.65 to 2.40) | 0.92 |
| ENT | −1.03 (−5.34 to 3.26) | 0.64 | −0.29 (−3.19 to 2.61) | 0.84 | −1.16 (−5.40 to 3.08) | 0.59 | 1.29 (−1.46 to 4.04) | 0.36 |
| Chest | −3.46 (−6.89 to −0.04) | 0.047 | −2.13 (−4.44 to 0.17) | 0.070 | −2.27 (−5.60 to 1.05) | 0.18 | −1.61 (−3.90 to 0.66) | 0.16 |
| Cardiac | −3.71 (−12.28 to 4.86) | 0.39 | −2.37 (−7.70 to 2.96) | 0.38 | 0.59 (−7.00 to 8.18 | 0.88 | 4.96 (−0.51 to 10.42) | 0.075 |
| Abdominal | 6.31 (−2.61 to 15.23) | 0.17 | 3.81 (−2.11 to 9.73) | 0.21 | −3.69 (−12.46 to 5.08) | 0.41 | 3.27 (−2.55 to 9.10) | 0.27 |
| Renal | −3.64 (−8.69 to 1.41) | 0.16 | −2.04 (5.46 to 1.37) | 0.24 | −0.42 (−5.36 to 4.52) | 0.87 | −3.36 (−7.15 to 0.43) | 0.081 |
| Nervous | −8.48 (−12.90 to −4.06) | <0.001 | −2.27 (−5.18 to 0.64) | 0.13 | −4.98 (−9.14 to −0.81) | 0.019 | −2.45 (−5.26 to 0.36) | 0.088 |
Table 5.
Multivariate model of association of patient characteristics with mental domains of the Short Form 36 questionnaire.
| Vitality | Social Functioning | Role Emotional | Mental Health | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | |
| Age | 0.0028 (−0.10 to 0.11) | 0.96 | 0.009 (−0.13 to 0.14) | 0.90 | −0.032 (−0.15 to 0.087) | 0.60 | −0.014 (−0.13 to 0.11) | 0.81 |
| Female | −2.50 (−5.14 to 0.13) | 0.062 | −1.75 (−5.12 to 1.61) | 0.31 | −2.01 (−4.98 to 0.97) | 0.19 | −3.28 (−6.29 to −0.27) | 0.032 |
| MPA | −0.44 (−4.05 to 3.16) | 0.81 | 0.35 (−4.30 to 5.01) | 0.88 | 2.17 (−1.95 to 6.29 | 0.30 | 3.84 (−0.29 to 7.98) | 0.068 |
| eGFR (per 10 ml/min) | 0.60 (0.10 to 1.11) | 0.018 | 0.56 (−0.084 to 1.20) | 0.088 | 0.32 (−0.24 to 0.89) | 0.26 | 0.24 (−0.33 to 0.83) | 0.41 |
| Organ Involvement | ||||||||
| Systemic | −3.73 (−10.12 to 2.66) | 0.25 | −6.66 (−15.14 to 1.81) | 0.12 | −7.24 (−14.55 to 0.07) | 0.052 | −3.34 (−10.73 to 4.06) | 0.38 |
| Cutaneous | 0.33 (−2.84 to 3.51) | 0.84 | −2.11 (−6.28 to 2.06) | 0.32 | 2.59 (−1.05 to 6.25) | 0.16 | 2.20 (−1.46 to 5.87) | 0.24 |
| Mucous Membrane/Eye | −1.78 (−4.81 to 1.24) | 0.25 | −3.31 (−7.30 to −0.67) | 0.10 | −0.52 (−3.97 to 2.93) | 0.77 | −0.20 (−3.72 to 3.32) | 0.91 |
| ENT | −0.94 (−4.29 to 2.40) | 0.58 | −0.14 (−4.46 to 4.17) | 0.95 | 2.80 (−0.97 to 6.58) | 0.15 | 3.89 (0.045 to 7.74) | 0.047 |
| Chest | −3.02 (−5.70 to −0.34) | 0.027 | −3.47(−6.94 to 0.0003) | 0.050 | −3.32 (−6.35 to −0.29) | 0.032 | −2.78 (−5.88 to 0.32) | 0.078 |
| Cardiac | 1.08 (−5.32 to 7.47) | 0.74 | 3.29 (−4.74 to 11.32) | 0.42 | −3.93 (−11.03 to 3.17) | 0.28 | −3.38 (−10.87 to 4.11) | 0.37 |
| Abdominal | 6.74 (−0.11 to 13.59) | 0.054 | 4.09 (−5.20 to 13.39) | 0.39 | 5.94 (−1.78 to 13.66) | 0.13 | 2.71 (−5.07 to 10.48) | 0.49 |
| Renal | 0.53 (−3.36 to 4.42) | 0.79 | −1.82 (−6.85 to 3.19) | 0.48 | −0.98 (−5.46 to 3.48) | 0.67 | 0.79 (−3.96 to 5.53) | 0.74 |
| Nervous | −0.95 (−4.32 to 2.41) | 0.58 | −0.94 (−5.23 to 3.34) | 0.67 | −1.91 (−5.70 to 1.88) | 0.32 | −2.81 (−6.70 to 1.08) | 0.16 |
In terms of organ involvement, General manifestations of AAV resulted in lower General Health (−6.50 points, 95% CI −12.07 to −0.93) scores but this did not meet the modified threshold for statistical significance (p=0.022). Neurological activity was associated with statistically and clinically significant lower Physical Functioning scores (−8.48, 95% CI −12.90 to −4.06; p<0.001) and there was a non-significant trend to lower Bodily Pain scores (−4.98, 95% CI −9.14 to −0.81; p=0.019). Other organ manifestations were not associated with differences in HRQoL scores.
Discussion
HRQoL is increasingly important to consider in the care of patients with AAV. Despite this, there are few studies demonstrating what features of AAV are important determinants of HRQoL. We have demonstrated in a large cohort that includes the full spectrum of severity of AAV that HRQoL, particularly in physical domains, is significantly reduced at the time of diagnosis. Neurological manifestations of AAV affect HRQoL most dramatically suggesting they may be an important therapeutic target to improve HRQoL.
HRQoL was substantially lower in our AAV patients than population norms. SF-36 scores in our patients also appeared lower than some other recent studies of HRQoL in patients with AAV (20). However, our study included only patients at the time of diagnosis while others typically measured HRQoL in a mixture of patients with active and inactive disease. The finding that physical domains of HRQoL were more affected than mental domains is also similar to other studies as was the lack of association between HRQoL and renal function, or diagnosis (21). Unique to our study, however, is the assessment of each organ system involvement and the finding that neurological activity most strongly affects HRQoL.
In our study, Role Physical scores were the lowest, a finding consistent with others, suggesting treatments that affect this domain will be of greatest value for improving HRQoL in patients with AAV (22). Also, we found a possible association between neurological manifestations and bodily pain which may have been an important determinant in other studies demonstrating neuropathic pain was a significant source of reduced HRQoL. However, unlike other reports, we did not find ENT activity associated with clinically significant reductions in any domain of HRQoL (5;7). In fact, those with ENT involvement appeared to have slightly better Mental Health scores compared to those without ENT involvement although this may well be a spurious finding. The discrepancy between ours and other studies may be due to fact that ours were newly diagnosed patients with active disease manifestation due to AAV as assessed by a physician at diagnosis. Other studies examined the associations between disease damage or persistent symptoms in patients with a pre-existing diagnosis of AAV (7;23). It is possible that persistent symptoms, which may be due to organ scarring or active disease, have a greater impact on patient’s HRQoL in the absence of acute illness and additional acute disease manifestations. This is consistent with the finding that chronic disease damage is associated with lower SF-36 scores in several domains (23;24).
Few organ manifestations were found to have a clinically and statistically significant association with reduced HRQoL in our study despite the finding that overall HRQoL was very impaired. This could be due to a relatively small contribution to reduced HRQoL from individual organ manifestations. Most patients present with a constellation of organ manifestations when diagnosed with AAV which together may result in significantly impaired HRQoL. Consistent with this was the observation that summing the contribution of the most common manifestations and adjusting for the average age of our patients suggests a similar reduction in PCS as was observed for the overall group. Alternatively, it is possible that generic HRQoL instruments are insensitive to the effects of many manifestations of AAV on HRQoL. Therefore, instruments like the SF-36 may be suitable to quantify overall changes in HRQoL but insufficiently sensitive to capture changes in specific areas of HRQoL that result from the treatment of very particular AAV symptoms. For example, although SF-36 may adequately quantify changes in HRQoL as patients transition from acute disease to complete remission it may be inadequate to quantify the impact of treating ENT symptoms on HRQoL. Studies that seek to measure improvement in specific areas of HRQoL may therefore be best served by using domain/symptom specific HRQoL instruments in addition to generic instruments as is recommended in other diseases (25).
Our study has several notable strengths. It is, to our knowledge, the largest study of HRQoL in patients with AAV and it covers the full spectrum of disease severity. As such, we are able to make more precise estimates of the effects of disease manifestations on HRQoL that are relevant to a broader scope of patients than previous studies. Further, all patients are newly diagnosed thus limiting confounding by duration of disease which may occur in cross-sectional studies. Finally the use of a generic instrument allows us to compare the HRQoL of our patients with patients with AAV in other studies and to patients with other diseases.
Our study must also be interpreted within the context of its limitations. A substantial number of patients did not complete the SF-36 and these patients tended to have more severe disease and be older than those who did complete the questionnaire. It is difficult to predict how this responder bias affects our results. However, it seems likely the patients who did not complete the questionnaire were the most ill with the most severe manifestations of AAV and had the lowest HRQoL. Their exclusion would likely result in an underestimate of the effect of severe manifestations of AAV such as neurological manifestations and severe renal disease. Our sample is also taken from randomized control trials which may limit how representative our patients are compared to a true inception cohort of patients with AAV. However, this limitation is unlikely to have affected the generalizability of the effect estimates of organ manifestations on HRQoL (i.e. the relative effects of a given organ manifestation on HRQoL are unlikely to differ substantially between trial and non-trial patients). Lastly, although patients were newly diagnosed, for some cases, disease activity had been present for some months prior to diagnosis, and some disease manifestations may have caused damage and then become quiescent prior to diagnosis. These potentially confounding effects were not assessed in this study.
In conclusion, patients with AAV have significantly reduced HRQoL at the time of initial diagnosis. Neurological involvement appears to be an important determinant of HRQoL and may be an important target for treatment and future research. However, additional research is required to determine which aspects of AAV determine HRQoL, in order to help direct future research and clinical care for these patients. Finally, our study highlights the need to evaluate HRQoL in clinical trials in AAV because the information it conveys is not encompassed by other, more traditional, vasculitis specific outcome measures.
Acknowledgments
MW is supported by the Canadian Institutes of Health Research. PM is supported in part through the Vasculitis Clinical Research Consortium which has received funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54AR057319), the National Center for Research Resources (U54 RR019497), the Office of Rare Diseases Research, and the United States Food and Drug Administration (FDA R01 FD003516-01).
Reference List
- 1.Bosch X, Guilabert A, Espinosa G, Mirapeix E. Treatment of antineutrophil cytoplasmic antibody associated vasculitis: a systematic review. JAMA. 2007;298(6):655–69. doi: 10.1001/jama.298.6.655. [DOI] [PubMed] [Google Scholar]
- 2.Mukhtyar C, Flossmann O, Hellmich B, Bacon P, Cid M, Cohen-Tervaert JW, et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann Rheum Dis. 2008;67(7):1004–10. doi: 10.1136/ard.2007.071936. [DOI] [PubMed] [Google Scholar]
- 3.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 5.Koutantji M, Harrold E, Lane SE, Pearce S, Watts RA, Scott DG. Investigation of quality of life, mood, pain, disability, and disease status in primary systemic vasculitis. Arthritis Rheum. 2003;49(6):826–37. doi: 10.1002/art.11471. [DOI] [PubMed] [Google Scholar]
- 6.Newall C, Schinke S, Savage CO, Hill S, Harper L. Impairment of lung function, health status and functional capacity in patients with ANCA-associated vasculitis. Rheumatology. 2005;44(5):623–8. doi: 10.1093/rheumatology/keh548. [DOI] [PubMed] [Google Scholar]
- 7.Srouji IA, Andrews P, Edwards C, Lund VJ. General and rhinosinusitis-related quality of life in patients with Wegener’s granulomatosis. Laryngoscope. 2006;116(9):1621–5. doi: 10.1097/01.mlg.0000230440.83375.4b. [DOI] [PubMed] [Google Scholar]
- 8.Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniene J, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349(1):36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 9.DeGroot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophilcytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52(8):2461–9. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 10.DeGroot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150(10):670–80. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 11.Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis [see comment] J Am Soc Nephrol. 2007;18(7):2180–8. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 12.Bowling A, Bond M, Jenkinson C, Lamping DL. Short Form 36 (SF-36) Health Survey questionnaire: which normative data should be used? Comparisons between the norms provided by the Omnibus Survey in Britain, the Health Survey for England and the Oxford Healthy Life Survey. J Public Health Med. 1999;21(3):255–70. doi: 10.1093/pubmed/21.3.255. [DOI] [PubMed] [Google Scholar]
- 13.Jenkinson C, Wright L, Coulter A. Criterion validity and reliability of the SF-36 in a population sample. Qual Life Res. 1994;3(1):7–12. doi: 10.1007/BF00647843. [DOI] [PubMed] [Google Scholar]
- 14.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 15.Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87(11):671–8. [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37(2):187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 18.Carlin JB, Galati JC, Royston P. A new framework for managing and analyzing multiply imputed data in Stata. The Stata Journal. 2008;8(1):49–67. [Google Scholar]
- 19.Royston P. Multiple imputation of missing values: update. The Stata Journal. 2005;5(2):1–14. [Google Scholar]
- 20.Carpenter DM, Thorpe CT, Lewis M, DeVellis RF, Hogan SL. Health-related quality of life for patients with vasculitis and their spouses. Arthritis Rheum. 2009;61(2):259–65. doi: 10.1002/art.24235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu N, Jones GT, Fluck N, MacDonald AG, Pang D, Dospinescu P, et al. Fatigue: a principal contributor to impaired quality of life in ANCA-associated vasculitis. Rheumatology (Oxford) 2010;49(7):1383–90. doi: 10.1093/rheumatology/keq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinhold-Keller E, Herlyn K, Wagner-Bastmeyer R, Gutfleisch J, Peter HH, Raspe HH, et al. Effect of Wegener’s granulomatosis on work disability, need for medical care, and quality of life in patients younger than 40 years at diagnosis. Arthritis Rheum. 2002;47(3):320–5. doi: 10.1002/art.10458. [DOI] [PubMed] [Google Scholar]
- 23.Exley AR, Carruthers D, Kitas G, Luqmani RA, Bacon P. Functional outcome vs. damage in systemic vasculitis. Arthritis Rheum. 1996;39(S9):S66, 238. [Google Scholar]
- 24.Seo P, Min YI, Holbrook JT, Hoffman GS, Merkel PA, Spiera R, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET) Arthritis Rheum. 2005;52(7):2168–78. doi: 10.1002/art.21117. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622–9. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]