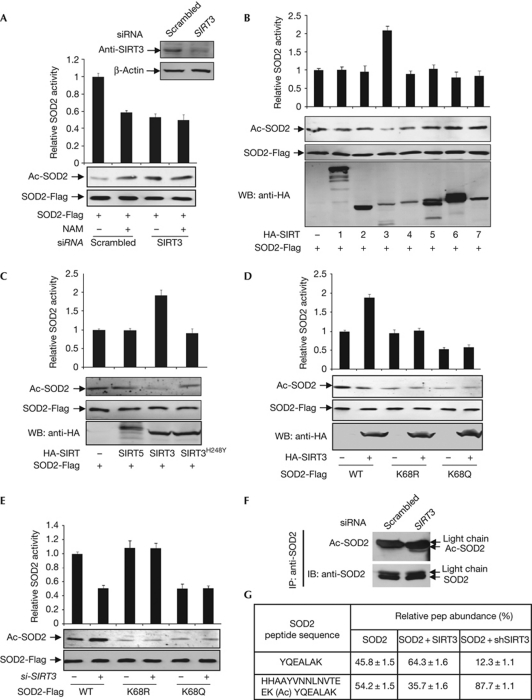
Figure 3.
SIRT3 deacetylates K68 and activates SOD2. (A) Knockdown of SIRT3 increases SOD2 acetylation and decreases SOD2 activity. Flag-tagged SOD2 was expressed in HEK293T cells, followed by transfection of siRNA oligo-targeting SIRT3. (B) SIRT3 activates SOD2. SOD2 was co-expressed in HEK293T cells with individual HA-tagged SIRTs, purified by Flag beads, followed by enzyme assay and western blotting. (C) Wild-type, but not catalytic mutant SIRT3 activates SOD2. SOD2 was expressed in HEK293T cells together with HA-tagged individual SIRTs as indicated. SOD2 proteins were purified by Flag beads. (D) SIRT3 does not activate K68 mutants of SOD2. Wild-type and mutant SOD2 were co-expressed in HEK293T cells with SIRT3 and purified by Flag beads, followed by enzyme assay and western blotting. (E) SIRT3 decaetylates K68 of SOD2. Acetylation levels and catalytic activities of SOD2 expressed and purified from HEK293T cells with or without SIRT3 knockdown were determined. (F) Knocking down SIRT3 increases endogenous SOD2 acetylation. Acetylation levels of endogenous SOD2 in HEK293T cells with or without SIRT3 knockdown were immunopurified and probed by acetyl lysine antibody. (G) Acetylation levels of K68 of SOD2 expressed in HEK293T cells with either SIRT3 overexpressed or knocked down were determined by iTRAQ. HA, haemagglutinin; HEK, human embryonic kidney; IB, immunoblotting; IP, immunoprecipitation; NAM, nicotinamide; siRNA, short-interfering RNA; SOD2; superoxide dismutase; WB, western blotting; WT, wild type.