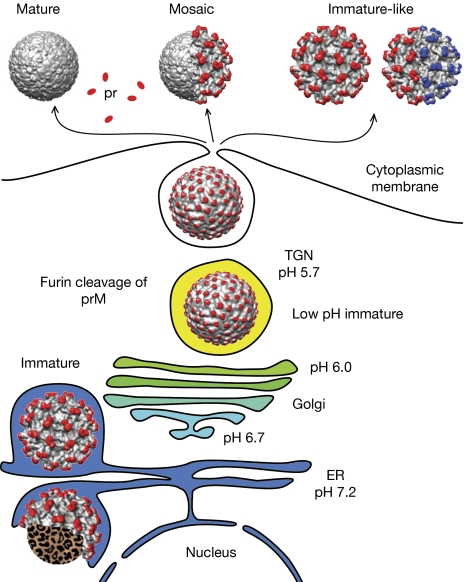
Figure 1.
A model of flavivirus maturation pathways. Immature particles form by budding into the endoplasmic reticulum. The envelopes of these particles have a single icosahedral symmetry. A conformational change of the virion occurs as the particles are transported to the acidic pH in the Golgi and TGN. The prM protein can be cleaved by the host protease furin after the conformational change, but not all prM molecules are cleaved. Depending on the fraction of prM cleaved, the particles might adopt mature, mosaic or immature-like conformations when released from cells. The cleaved precursor proteins are released from particles at neutral pH in the extracellular space. The immature-like particles only constitute approximately 3% of wild-type virions that are released from cells, but are abundant for the prM cleavage-deficient prR201A mutant. About 50% of the immature-like particles of the prR201A mutant had their envelopes organized into two regions, each of which correspond to an immature structure, but the two regions were symmetrically mismatched. The pr/prM proteins are shown in red/blue and the envelope proteins are shown in grey. The red and blue colours of prM indicate mismatched icosahedral symmetries. ER; endoplasmic reticulum; pr, precursor; prM, precursor membrane; TGN, trans-Golgi network.