Figure 1.
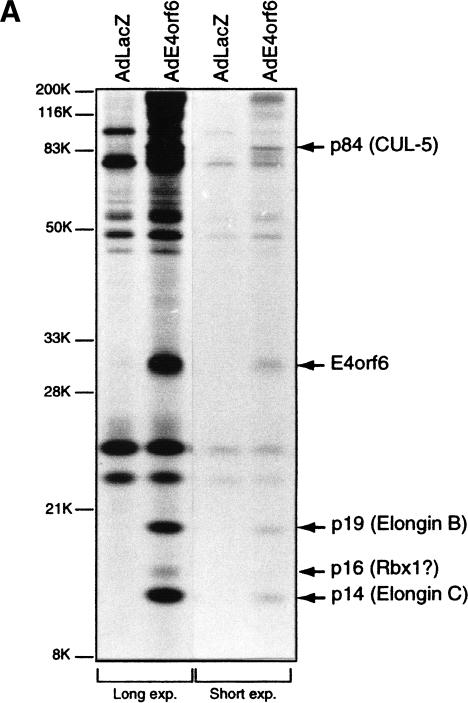
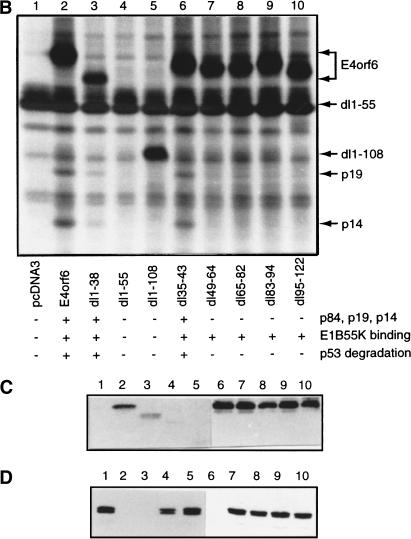
Analysis of E4orf6 complex formation. (A) Detection of E4orf6 complexes. Extracts from H1299 cells infected with AdLacZ or AdE4orf6 and labeled with 35S were immunoprecipitated with 1D5 anti-E4orf6 antibody followed by SDS-PAGE and autoradiography. Two exposures have been included: positions of migration of prestained standard size markers (left) and those of E4orf6 and coimmunoprecipitating proteins (right). Identities for these species were derived from mass spectroscopy analysis of tryptic peptides. (B) Analysis of complex formation using E4orf6 deletion mutants. H1299 cells were transfected with plasmid DNAs encoding wild-type or mutant E4orf6, and extracts were immunoprecipitated and analyzed as in A. Coimmunoprecipitation of p19 and p14 cellular proteins with E4orf6 has been summarized below as + and −. Binding of p84 showed a similar pattern, as determined from other exposures of the gel (data not shown). Binding of E4orf6 to E1B55K (shown in C) and p53 turnover experiments with E4orf6 and E1B55K (D) have also been indicated as + and −. (C) Complex formation between E4orf6 mutants and E1B55K. Extracts from H1299 cells transfected with plasmid DNAs expressing E1B55K (pCA14 HH55K) and wild-type or mutant E4orf6 proteins (pcDNA3E4orf6) were immunoprecipitated with anti-E1B55K antibody (2A6), and membranes were immunoblotted with anti-E4orf6 antibody (1807). (D) E4orf6/E1B55K-induced turnover of p53. H1299 cells were transfected with plasmid DNAs pcDNA3 p53wt (p53), pCA14 HH55K (E1B-55K), and pcDNA3E4orf6 (wild-type or mutant E4orf6), and extracts were analyzed by Western blotting using anti-p53 antibodies 1801 and 421.