FIGURE 4.
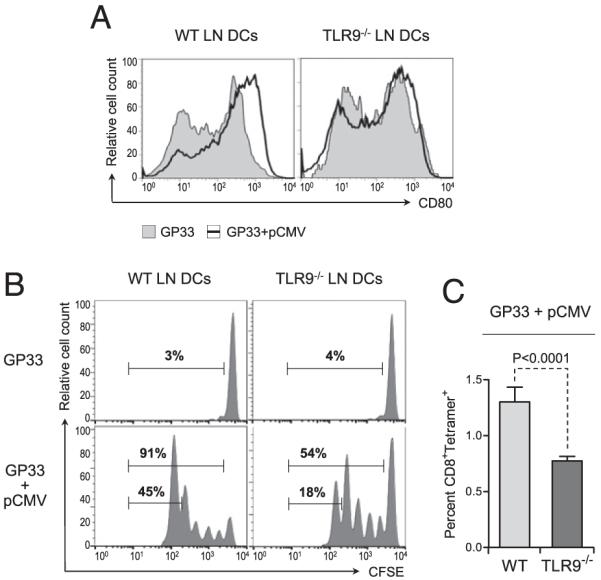
Plasmid DNA-mediated activation of DCs and subsequent priming of Ag-specific CD8+ T cell responses in vivo require TLR9 signaling. WT and TLR9−/− mice were injected with 2 μg GP33 alone or along with 50 μg pCMV in the tibial muscle. A, Twenty-four hours after injection, the popliteal LN was collected, and cell suspensions were stained for CD80 and analyzed by flow cytometry. Graphs represent CD80 expression on gated CD11c+ cells in the LN draining the muscle injected with GP33 and pCMV (open graph) or GP33 alone (shaded graph). Data are representative of five individual mice. B, Twenty-four hours after injection, the mice were adoptively transferred with 106 CFSE-labeled CD8+ T cells purified from the spleen of naive P14 mice. Forty-eight hours after transfer, proliferation of the injected T cells present in the popliteal LN was assessed by flow cytometry. Graphs represent CFSE dilution in gated CD8+ T cells in the LN draining the injected muscle. Numbers show the percentage of cells in the indicated gates. C, Twenty-four hours after injection, the mice were adoptively transferred with 106 CD8+ T cells purified from the spleen of naive P14 mice. Forty-eight hours after transfer, the percentage of GP33-specific T cells present in the popliteal LN was assessed by flow cytometry after staining with GP33 peptide/MHC tetramers. Bar graphs represent the average percentage (± SD) of tetramer+ CD8+ T cells in the LN draining the injected muscle for five individual mice per group. Statistical significance was calculated using a two-tailed t test.
