Figure 7.
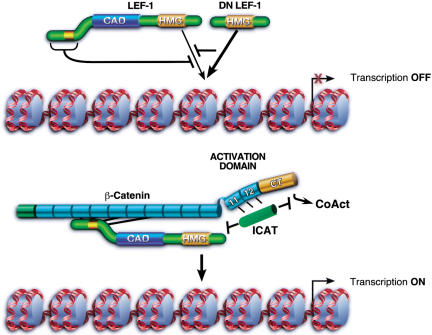
Chromatin-specfic effects of β-catenin on binding and transcriptional activation by LEF-1 in vitro. In the absence of β-catenin, Wnt-responsive genes are repressed by LEF/TCF–Groucho complexes, and binding of uncomplexed LEF-1 to chromatin is inhibited through amino-terminal residues. LEF-1 isoforms that lack the inhibitory amino-terminal domain bind chromatin more avidly and function as potent feedback inhibitors of Wnt signaling in vivo (dominant negative DN LEF-1). The interaction of the central ARM repeats of β-catenin with the N terminus of LEF-1 alleviates this inhibition, and LEF-1 binds cooperatively with its coactivator to chromatin. Optimal transactivation requires an activation domain located at the C terminus that may also include C-terminal ARM repeats 11 and 12. LEF-1–β-cat transcription can be selectively blocked in vitro by a dominant-negative fragment of β-catenin that spans the carboxyl terminus and ARM repeats 11/12, as well as by the physiological Wnt inhibitor, ICAT. ICAT, but not CT–ARM, can disrupt the interaction between LEF-1 and β-catenin. Moreover, ICAT can block transcription from complexes bound stably to chromatin, indicating that it may also disrupt interactions between β-catenin and downstream transcriptional coactivators (CoAct).