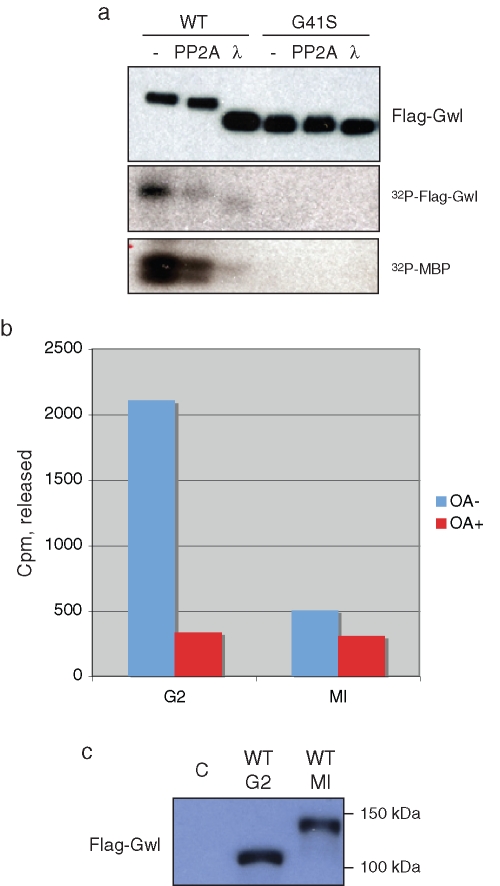
FIGURE 2:
Greatwall-bound PP2A is catalytically active. (A) Dephosphorylation and deactivation of Gwl. Oocytes were injected with mRNA encoding WT or G41S Gwl. After overnight incubation to allow protein expression, the oocytes were treated with progesterone. At GVBD, oocyte lysates were prepared and anti-FLAG bead immunoprecipitates incubated with either PP2Ac or lambda phosphatase as described in Materials and Methods. The precipitates were then washed and assayed for autophosphorylation or MBP kinase activity (bottom, autoradiographs), or blotted with anti-FLAG antibodies after SDS–PAGE (top), as indicated. (B) Phosphatase activity associated with Gwl. Oocytes were injected with mRNA encoding WT FLAG-tagged Gwl and incubated overnight. Some oocytes were then treated with progesterone to enter M phase. At GVBD, anti-FLAG beads were used to precipitate Gwl, and after washing, the beads were incubated with or without OA and 32P-labeled histone H1 prepared as described in Materials and Methods. The reaction was terminated by addition of 30% trichloracetic acid, and the counts per minute released were quantified by Cerenkov counting in a liquid scintillation counter. Similar results were obtained in several independent experiments. (C) Anti-FLAG immunoblot of the immunoprecipitates in B. C, control oocytes not injected with mRNA but subjected to anti-FLAG bead precipitation and assay. Activity associated with Gwl in B was corrected for background seen with control FLAG bead immunoprecipitates from uninjected oocytes.