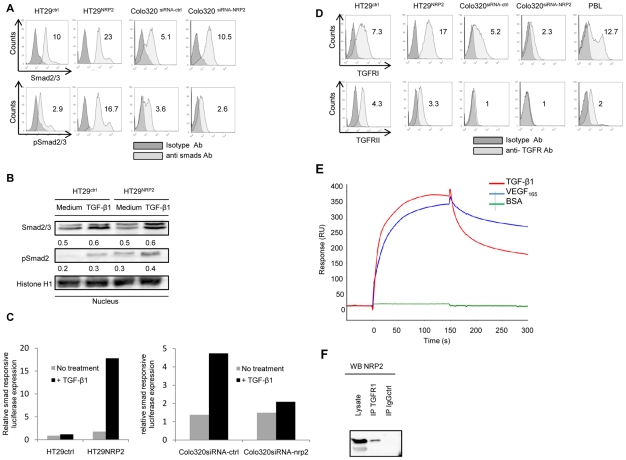
Figure 5. NRP2 acts as a TGF-β1 co-receptor.
A, smad2/3 and phospho-smad2/3 expression was assessed in HT29ctrl, HT29NRP2, Colo320siRNA-ctrl, Colo320siRNA-NRP2 by intra-cellular flow-cytometry. Relative Fluorescence Intensity (RFI) was calculated. B, Western-blotting experiments were performed on nuclear extracts of HT29ctrl and HT29NRP2 cultured in serum free conditions or with 10 ng/mL of TGF-β1 for 16 hours. Histone-H1 was used as a control of protein loading. Blotted proteins have been quantified with the BIO-1D advanced software and reported to the Histone-H1 level. While exogenous TGF-β1 is mandatory for smad2 phosphorylation detection in HT29, Smad2 is constitutively phosphorylated in HT29NRP2 cells without any previous exposition to exogenous TGF-β1. C, TGF-β1 Cignal reporter assay kit was used for the quantification of TGF-β1-induced smad2/3 signaling in HT29ctrl, HT29NRP2, Colo320siRNA-ctrl and Colo320siRNA-NRP2 cells. An up-regulation of approximatively 17 times of the smad response is observed in HT29NRP2 cells compared to HT29ctrl after TGF-β1 stimulation. At the opposite, smad dependent response is significantly decreased in Colo320 cells treated by siRNA targeting NRP2 compared to Colo320 cells treated by siRNA-control after TGF-β1 stimulation. Results are presented as the ratios between the firefly luciferase activity and the renilla luciferase activity (Ren/Luc) for each conditions. This experiment was realized 3 times, each time in triplicates. D, Expression of TGFRI and TGFRII in HT29ctrl, HT29NRP2, Colo320siRNA-ctrl, Colo320siRNA-NRP2 colon cancer cells. Relative Fluorescence Intensity (RFI) was calculated. Colo320 cells don't express TGFRII. E, Surface plasmon resonance studies were performed to explore TGF-β1 interactions with NRP2. Fc-NRP2 proteins were covalently grafted on a chemically activated self assembled protein chip. Injections of TGF-β1, BSA (Control -), VEGF (Control +) were performed at 250 nM in PBS-Tween 0.05%, before biacore analysis. These experiments were reproduced two times and showed a specific binding of TGFβ1 to Fc-NRP2 protein. F, NRP2 interacts with TGFRI in co-immunoprecipitaion experiments using HT29NRP2 cells.