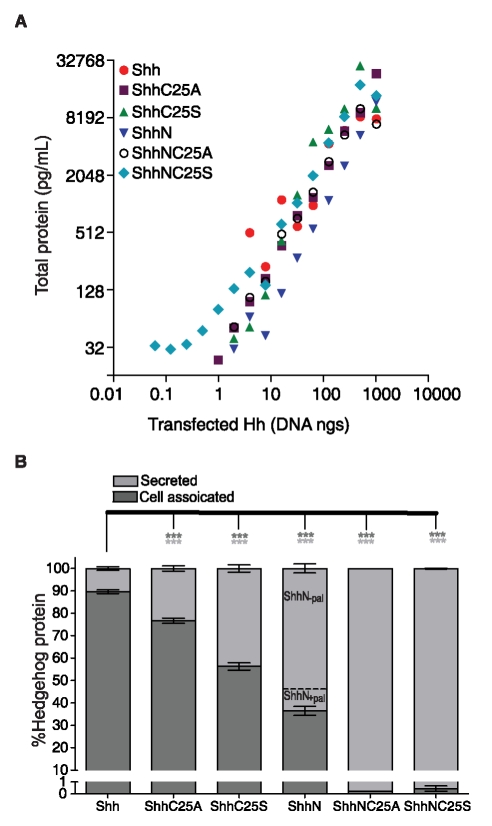
Figure 2. Shh lipid modifications enhance cellular association.
(A) Each of the constructs conferred dose dependent and similar expression levels of recombinant Shh protein. Shown are the sums of protein measurements in cell lysate and culture medium from NIH3T3 cells transfected with a range of cDNA for Shh, ShhC25S, ShhN, and ShhNC25S. (B) Precise quantification of recombinant Shh protein concentrations in either the cell lysate or culture medium revealed both cholesterol and palmitate modifications confer cellular association. Shown are the averages of Shh protein measurements from cells transfected with cDNA (62.5 ng to 500 ng in two fold increments). With both lipid adducts, 89.7% of Shh was recovered in the lysate and 10.3% was secreted in the culture medium. Less protein was measured in lysates from cells expressing Shh without a palmitate adduct, (76% for ShhC25A and 56.3% for ShhC25S). Examination of the culture medium from cells transfected with ShhN by HPLC (Figure S1) revealed the presence of two species of protein, one that was palmitoylated (ShhN+pal) and one that was not (ShhN−pal). Approximately 13% of secreted ShhN was palmitoylated (ShhN+pal). The portion of cell-associated ShhN (36.5%) represents the ShhN+pal species because in the absence of either lipid moiety, less than 0.5% of ShhNC25A and ShhNC25S protein was recovered from cell lysate. Protein measurements were performed in replicates of four (± s.e.m.) *** p<0.001 compared to Shh-transfected cells.