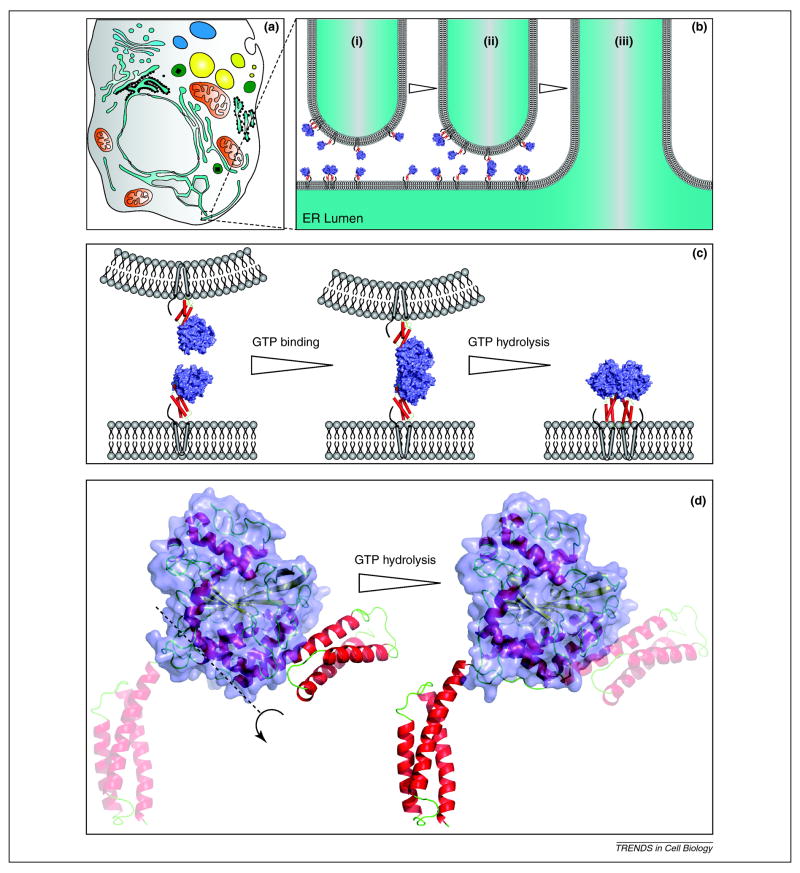
Figure 2. Proposed model for atlastin function in ER membrane fusion.
(A) A stylized eukaryotic cell depicting cytoplasmic organelles and the phycial relationship to the ER. A growing ER tubule approaching a peripheral ER tubule is highlighted in the dashed box. (B) A 50 nm diameter ER tubule is shown in proximity to a perpendicular ER tubule (i). Various atlastin molecules are shown to scale in both membranes. As the growing tube makes a close approach, form 2 atlastin monomers signal GTP binding and dimerization is favored (ii). GTP hydrolysis triggers the transition from form 2 to form 1 driving membrane fusion (iii). (C) Larger scale representations of the relevant interactions shown in panel B. (D) Structural models of Atl1crystal form 2 (left) and crystal form 1 (right). The proposed conformational changes that rotates the three-helix bundle out of the plane of the page about the axis marked by a dashed line results in form 2. The transparent three helix bundle on the left is the future location following GTP hydrolysis while the transparent three helix bundle on the right is the previous location after the conformational change. The GTPase domain is shown as a surface representation (blue) in all panels while the three-helix bundle segments are shown as red cylinders (B and C) or red helices (D). The location of the transmembrane domains is indicated by grey cylinders and the C-terminal tail as a thick black line in B and C. Atl1 crystal form 2 (3Q5E) and crystal form 1 (3Q5D) were rendered in pymol.