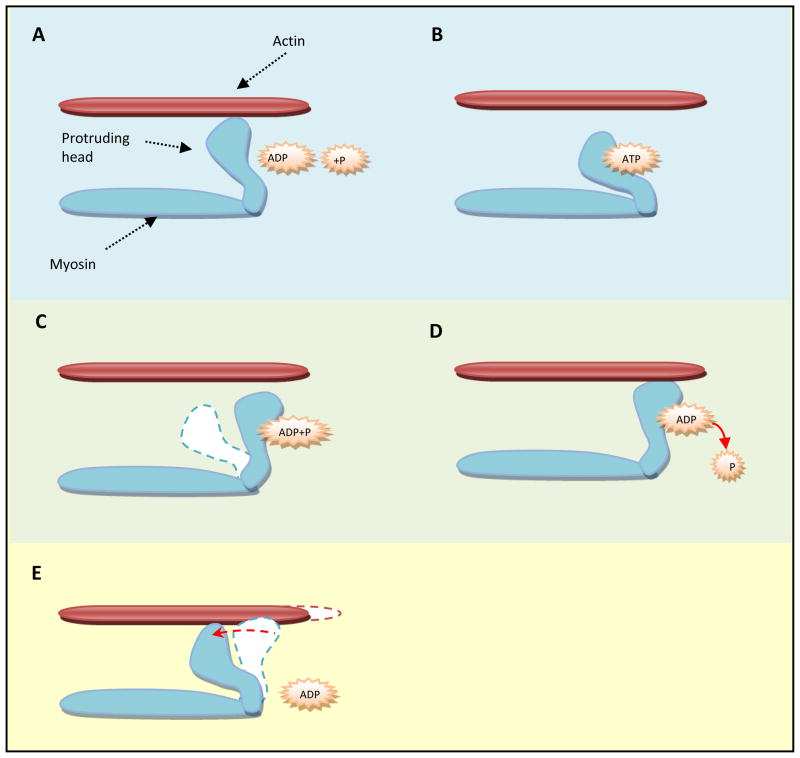
Figure 1.
Cross-bridge cycling in smooth muscle contraction. A. An initial rise in [Ca2+]i accompanied with increase in p-MLC20 via calmodulin-dependent MLCK activation causes activation of myosin ATPase and the formation of the ATP-myosin complex. B. ATP binding to protruding head in myosin structure causes a conformation change. C. As the ATP is hydrolyzed to ADP + P, the protruding head assumes an “erectile” position. D. When the P leaves myosin, the protruding head interacts with actin. E. These heads tilt and drag along the actin filament to produce movement. As ADP leaves the protruding head, the head tilts back towards the original conformation and drags along the actin filament to produce movement or smooth muscle contraction. Whether this phosphorylated cross-bridges cycling and contraction is very brief or sustained is determined by the balance between the forces that initiates the contraction (Ca2+/calmodulin/MLCK/p-MLC20) and dephosphorylation of p-MLC20 by MLCP. For the sustained contraction, it is important that dephosphorylation is inhibited by MLCP inhibition (via p-MYPT1) which is primarily mediated via RhoA/ROCK II or PKC activation. This has been explained further in the following figures.