Fig. 2.
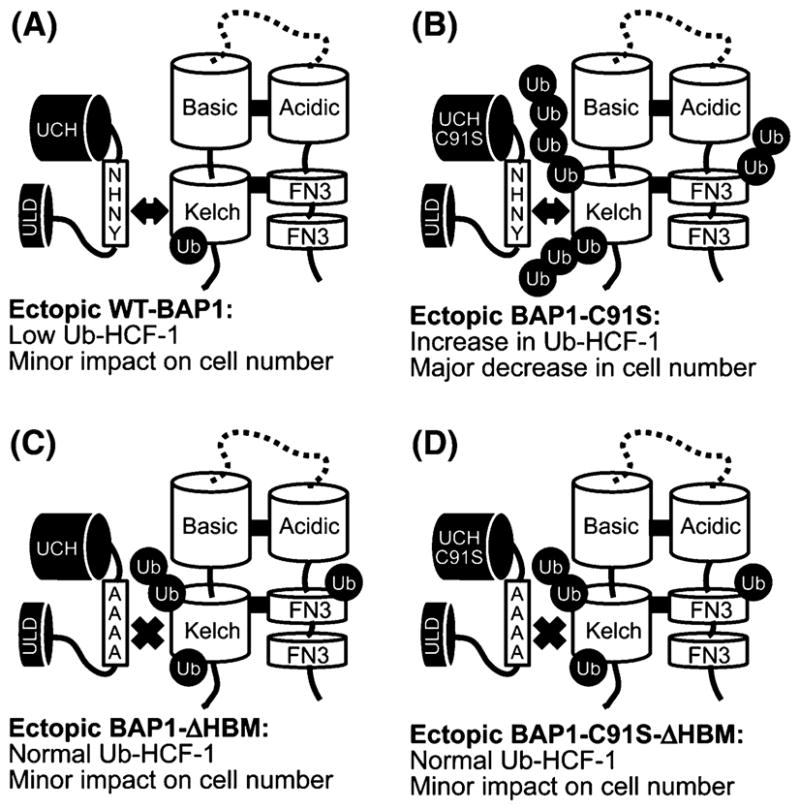
Model summarizing data from BAP1 overexpression studies. The report by Machida et al. [4] showed that knockdown of BAP1 levels by siRNA and overexpression of catalytically inactive BAP1 (C91S) both resulted in growth inhibition of MCF10a cells. To investigate the effects of BAP1 overexpression on the ubiquitination status of HCF-1, the authors co-overexpressed Flag-Kelch domain and HA-Ubiquitin and used anti-Flag immunoprecipitations to isolate the Kelch domain and any associated ubiquitin. Immunoblots revealed that WT BAP1 reduced the amount of ubiquitin that copurified with Flag-Kelch compared to cells lacking exogenous BAP1 (a). Expression of BAP1-C91S resulted in an increase in ubiquitin/Ub-Kelch (b) and thus the dominant negative effect on cell growth could be a product of inactive BAP1 saturating HCF-1 binding sites preventing endogenous, active BAP1 from deubiquitinating HCF-1. This model was tested by disrupting the BAP1/HCF-1 association, done by incorporating the ΔHBM alanine mutations with inactive BAP1 (BAP1-C91S-ΔHBM). When overexpressed the BAP1-C91S-ΔHBM protein resulted in a reduced amount of ubiquitin/Ub-Kelch that copurifies compared to BAP1-C91S (d). The BAP1-C91S-ΔHBM protein also rescued growth defects caused by overexpression of BAP1-C91S
