Fig 1.
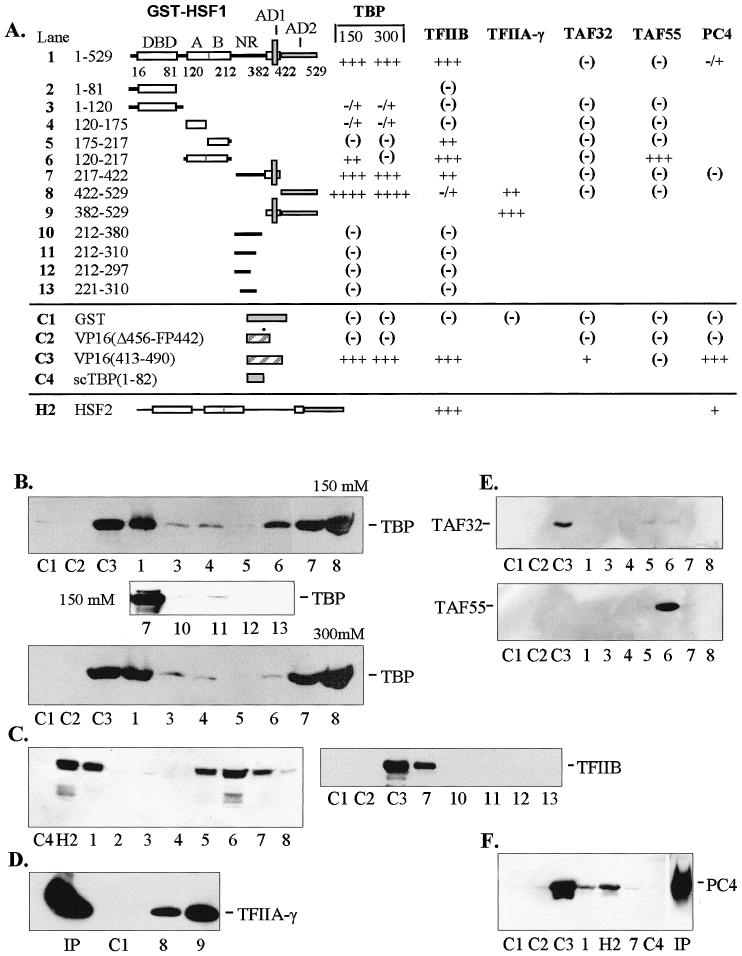
In vitro interactions between general transcription factors and human HSF1. (A) Diagrammatic summary of interactions between immobilized GST-HSF1 and TBP, TFIIB, TFIIA-γ, TAFII32, TAFII55, and PC4. The number of pluses (+) corresponds to the intensity of Western blot bands (panels B–F). Blanks indicate that the interaction was not tested. Positive and negative controls are designated C1 through C4 and represent constructs containing GST alone, VP16 mutant (Δ456-FP442), nonmutated VP16 (aa 413–490), and the N-terminus of yeast TBP (scTBP, aa 1–82), respectively. Construct numbers refer to lanes on Western blots shown in panels B through F. All transcription factors were expressed in E. coli and contained the T7 epitope at the N-terminus. All binding reactions were conducted with equal amounts of crude E. coli extracts containing full-length recombinant factors at 150 mM KCl unless otherwise indicated. (B) Interactions with human TBP at 150 and 300 mM KCl. With full-length GST-HSF1, approximately 80% on input TBP was bound under these conditions. (C) Interactions with TFIIB. Under these conditions, approximately 50% of input TFIIB was bound. (D) Interactions with TFIIA-γ. (E) Interactions with TAFII32 (top) and TAFII55 (bottom). (F) Interactions with positive coactivator PC4. Human HSF2 (H2) was tested with TFIIB and PC4. Input (Ip) lanes contained 10% of total present in binding assays. DBD, DNA-binding domain; A and B, hydrophobic regions A and B of the oligomerization domain (HR-A and HR-B); NR, negative domain; AD1 and AD2, activation domains 1 and 2
