It is well established that cross-presentation of donor cell-derived antigens is essential for the development of adaptive immune responses to many pathogens and malignant tumors. Long-lived protein but not short-lived proteins (SLiPs) expressed by tumor cells are normally cross-presented by host antigen presenting cells (APC). However, SLiPs were efficiently cross-presented when proteasome activity of tumor cells was inhibited. Interestingly, inhibition of proteasome function led to increased autophagy activity of tumor cells. We documented that efficient cross-presentation of tumor associated antigens or model antigens either expressed by tumor cells as short or long-lived proteins required functional autophagy function of antigen donor cells and isolated vesicles with enriched autophagosomes acted as a antigen ferry for highly efficient cross-presentation. Our report identified an important new role of autophagy and provided new insights into intelligent design of novel vaccines for cancers or infectious diseases.
Introduction
Cross-presentation of exogenous antigens by host professional antigen-presenting cells (APC) plays a pivotal role in the initiation and development of T-cell immune responses to tumor-associated antigens, including self or mutated self-antigens derived from tumor cells, and foreign antigens derived from infectious agents. Cross-presentation requires multiple steps that involve the antigens synthesis and compartmentalization in donor cells, packaging and delivery, and processing and presentation by MHC class I molecules on professional APC. The intricate pathways that lead to protein degradation and the formation of MHC I-peptide complexes inside the APC are well documented for both soluble and particulate antigens. However, much less is known about how cross-presentation is regulated by the protein degradation pathways in antigen-donor cells (ADC), including autophagy-mediated lysosomal proteolysis and proteasomal degradation. The exact nature or form of the antigens derived from donor cells at the time of delivery to the APC for cross-presentation is very controversial.
Autophagy and cross-presentation
After their synthesis, proteins “ready” for degradation are targeted either to lysosomes via autophagy or to proteasomes following ubiquitination. Very little is known about how proteins are partitioned between these two pathways; however, it is generally believed that most short-lived proteins (SLiPs), including the defective ribosomal products (DRiPs), are ubiquinated and degraded by proteasomes, while the long-lived proteins are sequestered in autophagosomes for lysosomal degradation. Under abnormal physiological conditions, i.e., when either pathway is defective, the degradation of proteins is shunted from one pathway to the other to protect cell survival. The proteasome-mediated protein degradation pathway plays a very important role in regulating cell signaling and providing peptides for MHC-I-restricted antigen presentation (direct presentation).
The autophagy pathway typically involves the disposal of misfolded or damaged proteins, or superfluous organelles, and the maintenance of cell survival under stressful conditions. The autophagy pathway in APC was shown to play a critical role in MHC-II-restricted antigen presentation of endogenous or exogenous antigens. Moreover, a functional autophagy pathway in phagocytes is required for Toll-Like Receptor-mediated recognition and activation of innate immune responses. Overall, the ever-increasing evidence in recent literature provides strong support to the nation that autophagy is plays a pivotal role in both innate and adaptive immune responses.
The degradation of these antigens in the tumor cells or infected normal cells, which alters the spectrum of antigens that are delivered to APC, could have an important impact on the efficiency of cross-presentation. Supporting this notion, it was shown previously that proteasome activity of ADC was beneficial or detrimental for cross-presentation depending on the model used. Surprisingly, the effect of the bulk degradation pathway, autophagy-mediated lysosomal proteolysis, on cross-presentation has not been studied. Using HEK 293T cells that expressed the long-lived ovalbumin antigen (V-TfR-GFP-OVA) or melanoma cells that endogenously expressed the gp100 tumor antigen as ADC, recently we examined whether macroautophagy of ADC could regulate the efficiency of cross-presentation of model and native tumor antigens to naïve T cells in vitro and in vivo. Modulation of autophagy with inhibitors (3-MA and NH4Cl) or inducers (rapamycin and starvation), or siRNA knock-down of the essential autophagy genes beclin 1 and Atg12, demonstrated that the early stages of autophagy, including initiation and elongation of the double-membrane structure, sequestration of cytosolic antigens, and formation of autophagosomes (the influx), but not late lysosomal fusion and degradation (the efflux), were required for efficient antigen cross-presentation of OVA or native gp100 antigen in vivo and in vitro.
DRibble as an antigen ferry for cross-presentation
Interestingly, autophagosomes, the efficient carriers of antigens for cross-presentation, were isolated not only from inside the cells but also from the culture media, suggesting that autophagosomes are released into the media by the cells when they were stressed. We also found HSP90-associated peptides, likely derived from short-lived proteins (SLiP) including defective ribosomal products (DRiP), likely component of the antigen pool in tumor cells are accumulated in these autophagosomes. Thus, autophagosomes, which are enriched by modulation of the bulk degradation pathways, can serve as a vessel to ferry a broad spectrum of tumor antigens, including intact proteins, DRiPs, and HSP-bound peptides, to APCs for efficient cross-presentation (Figure 1). We named these DRiPs-containing autophagosome-rich blebs DRibbles. Short-lived proteins including DRiPs, which may comprise a broader repertoire of TAA, gave rise to most peptide antigens presented by MHC I on tumor cells. Our recent report suggests this novel vaccine based on autophagosomes was more effective at mediating tumor regression than the current gold standard vaccine based on GM-CSF gene modified whole tumor cells. Therefore, efficient cross-presentation of these short-lived proteins may generate CD8 T cells that recognize a broader spectrum of tumor-associated antigens and kill tumor cells with a higher efficiency. The DRibble vaccine derived from tumor cells is being extensively investigated as a novel, potent vaccine platform for cancer immunotherapy in our laboratories. Based on these strong preclinical results, a clinical trial of autologous non-small cell lung cancer-derived DRibbles has begun at our institution.
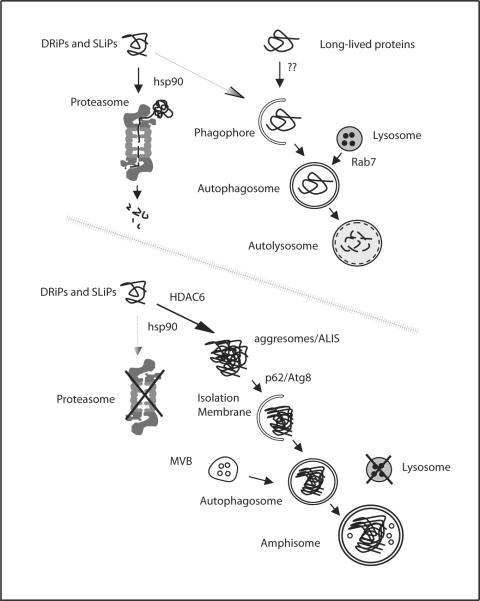
Figure 1. A working model of antigen accumulation inside autophagosomes with inhibition of both proteasomes and lysosomes.
Under normal culture conditions, the majority of DRiPs and SLiPs are ubiquitinated and targeted to proteasomes for their degradation, while long-lived proteins are targeted by autophagy to lysosomes for disposal. When both proteasomes and lysosomes are inhibited, SLiPs, DRiPs and misfolded proteins accumulate and form protein aggregates (ALIS/aggresomes), which then induce autophagy via p62 and Atg8/LC3 interaction. Peptide intermediates bound on hsp90 are encapsulated into autophagasomes and serve as immunogenic substrates for cross-presentation.
Acknowledgments
Our research was supported by the Providence Portland Medical Foundation, the American Cancer Society research scholar grant LIB-106810 (HMH), and Human Services Public Health Service grant R01 CA107243 (HMH), CA 080964 (BAF), and R43 CA121612 (SA) from NIH, and the National Natural Science Foundation of China grant/300771999 (LXW).
Footnotes
Disclosure of Potential Conflicts of Interest H-M. Hu and B.A. Fox have ownership interest in Ubivac, LLC. S. Aung is employed by Ubivac, LLC. The other authors disclosed no potential conflicts of interest.