Abstract
We observed how combined mechanical stimuli affect the proliferation and differentiation of pre-osteoblasts. For this research, a bioreactor system was developed that can simultaneously stimulate cells with cyclic strain and ultrasound, each of which is known to effectively stimulate bone tissue regeneration. MC3T3-E1 pre-osteoblasts were chosen for bone tissue engineering due to their osteoblast-like characteristics. 3-D scaffolds were fabricated with polycaprolactone and poly-L-lactic acid using the salt leaching method. The cells were stimulated by the bioreactor with cyclic strain and ultrasound. The bioreactor was set at a frequency of 1.0 Hz and 10% strain for cyclic strain and 1.0 MHz and 30 mW/cm2 for ultrasound. Three experimental groups (ultrasound, cyclic strain, and combined stimulation) and a control group were examined. Each group was stimulated for 20 min/day. Mechanical stimuli did not affect MC3T3-E1 cell proliferation significantly up to 10 days when measured with the cell counting kit-8. However, gene expression analysis of collagen type-I, osteocalcin, RUNX2, and osterix revealed that the combined mechanical stimulation accelerated the matrix maturation of MC3T3-E1 cells. These results indicate that the combined mechanical stimulation can enhance the differentiation of pre-osteoblasts more efficiently than simple stimuli, in spite of no effect on cell proliferation.
Keywords: bioreactors, cell differentiation, physical stimulation, tissue engineering, ultrasound
Introduction
Tissue engineering, which is a combined technology from cell biology to mechanical engineering and materials science, exploits living cells in a variety of ways to restore, maintain, or enhance tissues and organs (Langer and Vacanti, 1993; Griffith and Naughton, 2002). To engineer living tissues in vitro, cultured cells need to grow on a biodegradable scaffold because this scaffold is able to guide cells into forming functional engineered tissues in a three-dimensional (3-D) culture environment (Lan et al., 2009). Technologies to fabricate optimal scaffolds have been developed in various ways, including solid free-form fabrication using computer-aided design (Lee et al., 2008; Park et al., 2010).
Another rising issue in the field of tissue engineering is the construction of in vitro environments comparable to native tissue for growing cells or tissues. Because living tissues are exposed to a multiplicity of internal and external environments, these environments are believed to be capable of influencing the regeneration of tissues or organs (Mauney et al., 2004). Such environments involve biochemical, biophysical (e.g., pH, gas exchange, humidity, and temperature), and mechanical stimuli inside as well as outside the body. While biochemical stimuli have been researched extensively, mechanical stimuli have not, although mechanical environments generated by ultrasound or cyclic strain have been studied by many researchers, as these stimuli were found help bone wound healing (Rubin et al., 2001; Davisson et al., 2002; Yang et al., 2005; Meyer et al., 2006).
Cyclic strain includes repeatable tensile strain as well as cyclic compressive strain. It was induced from a bio-mimicking concept for the regeneration of cartilage and bone because these tissues are constantly exposed to cyclic strain when bodies are moving. Pre-osteoblast MC3T3-E1 cells express specific bone-related genes, such as bone morphogenic protein-2 (BMP-2), runt-related transcription factor-2 (RUNX2), and MAD homolog 5 (SMAD5), when strain is applied (Rath et al., 2008). Moreover, cyclic strain can make bone marrow stromal cells differentiate to osteoblasts with a higher level of alkaline phosphatase and osteopontin (Mauney et al., 2004).
Ultrasound is cyclic sound pressure with a frequency greater than 20 kHz, which is the upper limit of human hearing. Since it was first demonstrated that continuous-wave ultrasound could stimulate the formation of bone callus in rabbits (Corradi and Cozzolino, 1952), ultrasound has been applied to various fields for treatment and diagnosis. Especially low intensity ultrasound stimulation, typically delivered at the diagnostic intensity level of less than 50 mW/cm2, accelerates the healing of neural tissue, cartilage, tendon, and bone (Recher et al., 1998; Rubin et al., 2001). Moreover, low-intensity (30 mW/cm2) ultrasound has been shown to increase prostaglandin-E2 production through the induction of cyclo-oxygenase-2 mRNA in mouse (Kokubu et al., 1999). Additionally, low-intensity ultrasound can improve ECM synthesis (Saito et al., 2004).
However, most researchers are still investigating the effects of single mechanical stimuli, such as compression, shear stress, and ultrasound, even though native cells or tissues are inherently exposed simultaneously to multiple stimuli. No studies could be found on how cells can be affected when two or more stimuli are applied simultaneously. Therefore, we set out to develop a novel bioreactor system that allows controlled mechanical cyclic strain with simultaneous ultrasound treatment to cells on a scaffold. In this study, we further characterized the effects of combined stimulation, specifically ultrasound and cyclic strain, on pre-osteoblast MC3T3-E1 cells in 3-D environments in vitro using the developed bioreactor system. To provide a 3-D environment for the cells, a scaffold was fabricated by salt leaching, which provides a porous structure consisting of a mixture of polycaprolactone (PCL) and poly-L-lactic acid (PLLA). This PCL/PLLA mixture was chosen because it was considered to give the cells moderately elastic 3-D surroundings, such that mechanical stimuli were transferred directly.
Results
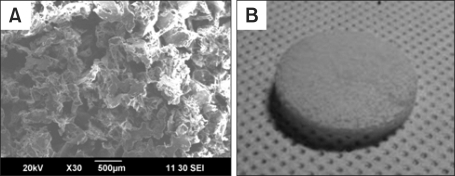
The PCL/PLLA scaffolds were well fabricated, as illustrated in Figure 1. The average pore size measured from SEM images was in the range of 300-350 µm. Mechanical testing confirmed that the scaffolds deformed elastically up to 0.58 mm (approximately 19.3% of the original thickness of the scaffold, 3 mm) of compressive displacement.
Figure 1.
Fabricated PCL/PLLA scaffold. (A) SEM image magnified ×30; (B) whole image of the disk-shaped scaffold.
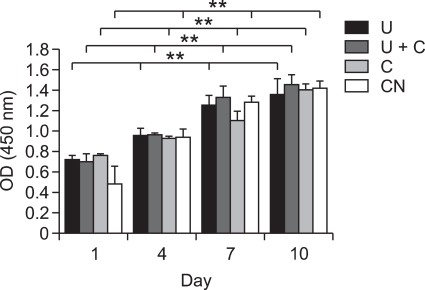
The effect of mechanical stimuli on the proliferative activity of MC3T3-E1 cells was assessed by CCK-8 assay. After seeding the cells on the scaffolds, they were exposed to various mechanical stimuli for 20 min/day. Cells were analyzed at 1, 4, 7, and 10 days after seeding. As depicted in Figure 2, the mechanical stimuli did not affect cell proliferation significantly.
Figure 2.
Proliferation of MC3T3-E1 cells on PCL/PLLA scaffolds according to various mechanical stimuli determined using CCK-8 assays. Results are presented as average ± standard deviation (n = 3). U, ultrasound; U+C, combined stimulation; C, cyclic strain; CN, control. Double asterisks (**) represent statistically significant differences at P < 0.01.
In the presence of ascorbic acid and β-glycerophosphate, pre-osteoblasts undergo a process of differentiation to osteoblasts marked by a decline in proliferation and the expression of osteogenic genes (Kreke et al., 2005).
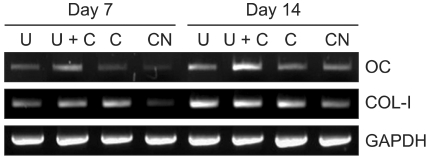
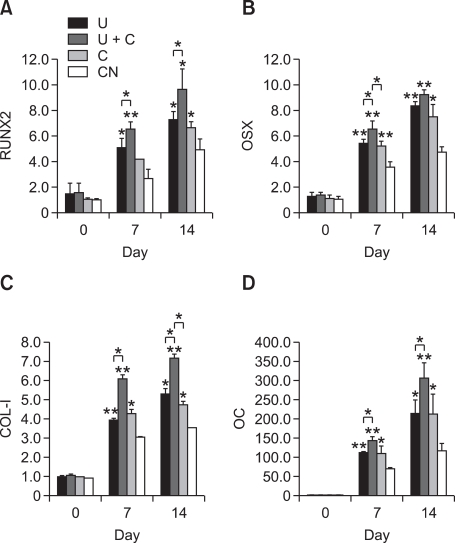
To further investigate the effect on differentiation of pre-osteoblasts of various mechanical stimuli, the expression of osteogenic mRNA (COL-I, OC) was assessed by RT-PCR. As shown in Figure 3, the expression levels of these genes in the mechanically stimulated groups were generally higher than in the control group at both day 7 and day 14. Higher expression levels of COL-I was observed in the mechanically stimulated groups. In particular, the expression levels of OC in the cells treated concurrently with ultrasound and cyclic strain were the highest among all experimental groups tested. These results are also shown quantitatively in real time PCR data. Figure 4 indicates ultrasound and cyclic strain can enhance the expression level of osteogenic markers. COL-I, OC, RUNX2, and OSX were highly expressed at day 7 and 14 when cells were mechanically stimulated. Moreover, the stimulated group using cyclic strain and ultrasound simultaneously (U+C) shows the highest gene expression in all cases. The U and C groups had similar levels.
Figure 3.
Gene expression of MC3T3-E1 cells on PCL/PLLA scaffolds according to various mechanical stimuli as analyzed by RT-PCR at days 7 and 14. OC, osteocalcin (58℃); COL-I, collagen type I (65℃); GAPDH, Glyceraldehyde 3-phosphate dehydrogenase (65℃).
Figure 4.
The levels of gene expression measured at day 0, 7, and 14 using real-time PCR. (A) RUNX2; (B) OSX; (C) COL-I; (D) OC. Single asterisks (*) denote statistically significant differences relative to the control at P < 0.05; double asterisks (**) indicate statistically significant differences relative to the control at P < 0.01.
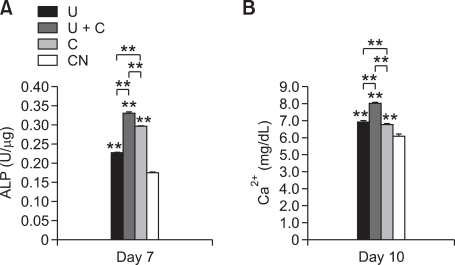
The ALP activity of each group was measured at day 7. Figure 5A shows that U+C can increase ALP activity compared with U, C, and CN. In Figure 5B, U+C also induced the highest calcium concentration among all groups at day 10. These data support mechanical stimuli accelerate osteogenic differentiation. Additionally, cyclic strain and ultrasound have synergistic effects on osteogenic differentiation of pre-osteoblasts.
Figure 5.
(A) ALP activity and (B) calcium concentration. Double asterisks (**) indicate statistically significant differences relative to the control, P < 0.01.
Discussion
In this study, we hypothesized that combined stimuli can have stronger effects on both cell proliferation and differentiation than a simple mechanical stimulus. To demonstrate this, a novel bioreactor system was developed for stimulating cells on 3-D PCL/PLLA scaffolds by generating cyclic strain and ultrasonic waves. The properties of the ultrasonic transducers were measured with a hydrophone, so they could be located such that the transferred intensity was easy to predict. The designed bioreactor contained a commercially available well plate for convenient use. Additionally, the bioreactor was so compact that it could be placed in a typical incubator or on a clean bench. To provide a 3-D environment for cells during the experiments, a salt leaching method was used to fabricate of PCL/PLLA scaffolds.
We hypothesized that combined stimuli could increase cell proliferation because several studies had reported that each stimulus has a positive effect to enhance cell proliferation in the specific range of intensity or strain (Rubin et al., 2001; Davisson et al., 2002; Yang et al., 2005; Meyer et al., 2006). The CCK-8 results, however, indicated no significant differences among groups when cells on 3-D PCL/PLLA scaffolds were exposed to cyclic strain, ultrasound, or both simultaneously in our system. However, we found that cells in all groups proliferated as time went by. Therefore, we think that mechanical stimuli did not affect the proliferation of pre-osteoblasts.
Many genes are related to bone formation. We chose COL-I, OC, RUNX2, and OSX among the various genes available. OC, known as bone gamma-carboxyglutamate (gla) protein, is a marker of bone formation and a vitamin K- and vitamin D-dependent protein produced by osteoblasts and the most abundant and widely studied of the non-collagenous proteins in bone (Lee et al., 2000). The other gene we examined was COL-I, which represents by far the most predominant collagen in the ECM of bone.
Together, the expression levels of these genes can reflect three stages of differentiation to osteoblasts (Owen et al., 1990). Pre-osteoblasts secrete ECM components and reach the calcification stage after the proliferation stage. ALP can be highly expressed during the period, such that the cell status changes from the proliferation stage to ECM secretion stage. OC and osteopontin are expressed thereafter when the cell status reaches the calcification step; both are involved in binding calcium ions and hydroxyapatite during the mineralization processes. Additionally, the osteogenic regulators RUNX2 and OSX are indispensable for osteoblast-specific genes including the osteocalcin gene (Ali et al., 2007; Lee et al., 2010).
Results in Figure 4 indicate that mechanical stimuli can enhance gene expression, when we compare the levels of day 0 with those of day 7 and 14. Even though levels of CN were increased due to the treatment of the osteogenic medium, levels of mechanically stimulated groups were higher than those of CN. Simple stimuli (U and C) or combined stimulation (U+C) could enhance pre-osteoblast differentiation to osteoblasts according to gene expression levels related to bone formation. The higher level of COL-I and OC (U+C) at 7 and 14 days after cell seeding compared to either single stimuli or no stimulation indicate that the cells were in the early stage of calcification. Even single mechanical stimulation up-regulated osteogenic transcription factors, such as RUNX2 and OSX, in the real time PCR. In particular, combined stimulation (U+C) caused the highest increase in RUNX2 and OSX. This over-expression of transcription factors in mechanically stimulated groups provides evidence that stimulation by ultrasound or cyclic strain promoted MC3T3-E1 differentiation. In particular, the combination of ultrasound and cyclic strain was the most effective.
The ALP activity result supports the data in the mRNA level. The ALP activity detected at day 7 after seeding indicates combined mechanical stimulation composed of cyclic strain and ultrasound enhances osteogenic differentiation. The calcium content result also supports this data. The calcium content was higher in U+C than in U and C. This higher function of differentiated pre-osteoblasts in U+C shows that cyclic strain and ultrasound have synergistic effects on osteogenic differentiation.
In conclusion, we showed that combined stimulation composed of cyclic strain and ultrasound can enhance MC3T3-E1 differentiation to osteoblasts in three-dimensional culture, whereas cell proliferation is not affected significantly. We demonstrated for the first time that combined stimulation can significantly up-regulate COL-I, OC, RUNX2, and OSX, thereby stimulating the differentiation of osteoblasts and bone formation. Our study will expand in the future to evaluate more complex stimuli to mimic the real environments of living organisms. Primary stem cells are considered to be more suitable for this investigation instead of a cell line, so they will be investigated in future research. In addition, although mechanical stress has been well established to induce osteoblast differentiation and osteogenesis both in vivo and in vitro, the mechanism by which mechanical stress induces these effects remains largely unknown. We plan to elucidate this mechanism.
Methods
Bioreactor
The bioreactor system has two main abilities: stimulating cells by cyclic strain and ultrasound (Supplemental Data Figure S1). The overall system consisted of a function generator, stage controller, power amplifier, and oscilloscope (Supplemental Data Figure S2A). The bioreactor containing samples is shown in the left side of the clean bench (Supplemental Data Figure S2B and C).
Cyclic strain was performed using a 1-axis motorized stage (AM1-0620-3S; Micro Motion Technology, Incheon, Korea) for precise control of the applied strain field. The stage had 0.002 mm/pulse (full step) resolution and could be operated by computer to control conditions such as cycles, velocity, acceleration, delay time, and displacement. A commercial well plate was used for cell cultures for convenience; Several samples could be individually treated by replacing the well plate in one bioreactor. Moreover, polytetrafluoroethylene (PTFE, Teflon®) strain bars were used, to stimulate cells directly on the scaffolds. These were cylinders with a diameter slightly smaller than that of a well of the well plate. These strain bars were directly connected to the motorized stage. Thus, their motion could be controlled by the computer. When the strain bars traveled along the z-axis over the cells on the scaffold with the preset parameters, the cyclic strain was applied directly to the cells.
Ultrasound stimulus was applied using transducers fed the signal from the function generator after power amplification. Three ultrasonic piezoelectric transducers (Fuji Ceramics Corp., Tokyo, Japan), which have a frequency of 1.0 MHz, were located at the bottom of the water tank (138 × 95 × 75 mm, l × w × h) used for the ultrasound stimulation of cells. A distilled water medium in the tank allowed efficient transmission of acoustic power. In the design process, the distance between the transducer and well plate was determined according to the ultrasonic beam profile, as detailed below. The power amplifier was used to increase the intensity because the function generator alone could not generate power in the range of 30-100 mW/cm2.
Acoustic analysis
We measured the axial beam profile of the transducers to set the distance from the scaffolds to the transducers and to predict the intensity of ultrasound that the cells would receive. It is important that the cells be positioned in the far field to provide them with a predictable and stable ultrasonic wave because a nonuniform beam intensity exists in the near field (area of nondivergence) and a uniform beam is present in the far field (area of divergence) (Hedrick et al., 1995). A hydrophone was used to measure the acoustic wave pressure generated by ultrasonic transducers, as described in Supplemental Data Figure S3A and schematically in Supplemental Data Figure S3B. Here, the width of the wave is measured radially, and the length is the distance it travels axially. We used the spatial average temporal average (SATA) intensity, which is the total power of the beam divided by the beam area and averaged over the repetition periods. The axial beam profile of intensity measured by the hydrophone is shown in Supplemental Data Figure S4. The intensity values at the center of the transducer are plotted in Supplemental Data Figure S5. This data indicates the far field begins at approximately 45 mm from the transducer, where the peak point occurs just prior to the intensity decreasing gradually. Thus, the bioreactor was designed so that the transducers were at least 45 mm from the samples.
Scaffold fabrication
Poly (L-lactic acid) (PLLA) was supplied by Lakeshore Biomaterials (Birmingham, AL). The weight average molecular weight (MW) of PLLA was 236 kDa (inherent viscosity, 1.9 dl/g), and the Mw of poly(caprolactone) (PCL; Sigma-Aldrich, St. Louis, MO) was 65 kDa. The 3-D polymer scaffolds were prepared using a particulate leaching technique (Lu et al., 2000). PLLA polymer (1 g) and PCL polymer (3 g) were dissolved in chloroform at room temperature (5-7%, w/v) with magnetic stirring, and sieved NaCl (300 µm particle size) was added to the polymer solution (weight ratio of NaCl to total polymer was 10:1). The dispersion was cast in a mold made of Teflon® (30 mm diameter, 3 mm thick) and the polymer/salt/solvent mixture was air-dried for 24 h under atmospheric pressure to remove solvent. After wetting the solidified mixture with cold ethanol, the resultant polymer/salt composite was immersed in excess distilled water for 48 h (water was changed every 4-6 h) to leach out the salt, and finally air- and freeze-dried. The pore size of the fabricated scaffolds was measured by scanning electron microscope (SEM: JSM-6390, JEOL, Tokyo, Japan). Mechanical testing was performed using a universal testing machine (3345, Instron, Canton, MA).
Cell culture
Murine MC3T3-E1 pre-osteoblasts, purchased from RIKEN Cell Bank (Tsukuba, Japan), were maintained in alpha minimum essential medium (α-MEM; Gibco, Invitrogen, Grand Island, NY) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco, Invitrogen) and 1% (v/v) streptomycin at 37℃ in an atmosphere of 95% humidified air and 5% CO2. The spontaneous differentiation into osteoblasts and mineral deposition of MC3T3-E1 cells were induced by adding, 10 mM β-glycerophosphate (Sigma-Aldrich) and 50 µg/ml ascorbic acid (Sigma-Aldrich) to the culture medium. For each subculture, the cells were washed with phosphate-buffered saline (PBS; Gibco, Invitrogen) and incubated with trypsin-EDTA solution (0.25% trypsin, 1 mM EDTA; Gibco, Invitrogen) for 5 min at 37℃ to detach the cells, and fresh medium was added at room temperature to inhibit the effect of the trypsin-EDTA solution. The cells were washed with fresh medium using a pipette and then isolated by centrifugation. After being resuspended in fresh medium, the cells were reseeded on culture dishes with 3-times larger area than before. That is, each time the cells were detached from a culture dish, we reseeded them in three culture dishes. After acquiring enough cells, 1 × 106 cells were seeded onto each scaffold. The culture medium was changed twice per week.
Exposure of cells to various mechanical stimuli
Experimental design
Three stimulated groups and a control group were prepared to determine the effect of combined stimulation of cyclic strain and ultrasound. The three stimulated groups were ultrasound, combined stimulation (ultrasound and cyclic strain simultaneously), and cyclic strain (Supplemental Data Table S1). Cell proliferation was examined at 1, 4, 7, and 10 days and differentiation testing was carried out at 7 and 14 days after seeding cells on the scaffolds.
Stimulation
Three ultrasound transducers at the bottom of the water tank could generate acoustic waves within the intensity range of 30-100 mW/cm2. After warming up the ultrasound equipment, cell-seeded scaffolds were placed on a 6-well plate located on the plate holder of the water tank. Because the well plate was floating directly on the water surface, the travel distance of ultrasound equaled the height from the transducer to the well plate. This was important when we estimated the ultrasonic intensity that the cells would receive. Air bubbles were removed between the plate and the water surface since they can block ultrasonic waves. Ultrasound was used to stimulate cells on the scaffold at a frequency of 1.0 MHz and intensity of 30 mW/cm2, in a continuous sine wave for 20 min daily.
Cyclic strain was at a frequency of 1.0 Hz, with 10% strain of the thickness of the scaffold. The sterile Teflon® stimulators were placed just on the top of the surface of each scaffold. Combined stimulation was applied with the same frequency and amplitude as described above for ultrasound and cyclic strain. The duration of each stimulus was 20 min per day. The cells on the scaffolds were incubated in a humidified incubator at 37℃ under 5% CO2 atmosphere when they were not being stimulated.
CCK-8 assay
Cell proliferation was determined using a Cell Counting Kit-8 (CCK-8; Dojindo Laboratory, Kumamoto, Japan). CCK-8 can detect cell viability using tetrazolium salt, WST-8[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2, 4-disulfophenyl)-2H-tetrazolium, monosodium salt], which produces a water-soluble formazan dye upon bioreduction in the presence of an electron carrier. Briefly, CCK-8 solution was mixed with serum-free α-MEM in a ratio of 1:10. After cell-seeded PCL/PLLA scaffolds were transferred into new well plates, serum-free α-MEM containing CCK-8 solution was added. After incubation for 3 h in a humidified 5% CO2 atmosphere, the formazan solution in the cell-seeded scaffolds was extracted by pipetting gently several times. The absorbance of the colored formazan was measured using a microplate reader (FLUOstar OPTIMA, BMG Labtechnologies, Offenburg, Germany) at 450 nm.
RT-PCR
The expression of osteogenic mRNA in pre-osteoblast MC3T3-E1 cells was investigated by RT-PCR (reverse transcription polymerase chain reaction). Total RNA was extracted from cells in the PCL/PLLA scaffolds using Trizol™ reagent (Invitrogen, Groningen, Netherlands). 2 µg of total RNA was used for cDNA synthesis using the Superscript™ first-strand synthesis system (Invitrogen) according to the manufacturer's instructions. Next, 2 µl of the synthesized cDNA was used for PCR with the primers listed in Supplemental Data Table 2. The specificity of the primer sequences was verified by BLAST search (National Center for Biotechnology Information). PCR was conducted at 95℃ for 15 s, 60℃ for 30 s, and 72℃ for 1 min. The annealing conditions were 58℃ for 1 min (OC), 65℃ for 1 min (COL-I), or 65℃ for 1 min (GAPDH). The products were separated by 2% agarose gel electrophoresis and visualized by ethidium bromide staining with UV light using a photo-documentation system (ChemiImager 5500; Alpha Innotech, San Leandro, CA).
Quantitative real-time PCR was performed according to the instructions provided for the ABI StepOnePlus system (Applied Biosystems, Foster City, CA) with the SYBR® Green PCR Mater Mix assay (Applied Biosystems, Foster City, CA). The increase in reaction products during real-time PCR was monitored by measuring the increase in fluorescence caused by the binding of SYBR Green to double-stranded DNA directly during PCR cycles. The expression values of target genes were standardized against those of GAPDH. The annealing condition was 60℃ for 1 min (COL, OC, GAPDH, RUNX2, and OSX).
ALP activity
The MC3T3-E1 cells cultured on the scaffold for 7 days were rinsed twice with PBS. Next the cells were lysed in 1mL lysis buffer (RIPA buffer, upstate, Temesula, CA). After centrifugation for 10 min at 12,000 rpm and 4℃ to remove cell debris, 300 µl of the supernatant was added to the pNPP at 37℃ for 30 min in a humidified 5% CO2 atmosphere. The reaction was stopped by the addition of 2 N NaOH. Absorbance was measured at 405 nm using a microplate reader. ALP activity was normalized to the total protein content of each scaffold associated with the cell surfaces and matrix using a commercially available protein assay kit (Pierce® BCA Protein Assay Kit; Thermo Scientific, Waltham, MA).
Calcium content assay
Cell lysate was extracted in lysis buffer (RIPA, upstate, Temecula, CA) and calcium content was measured using QuantiChrom Calcium Assay kit (BioAssay Systems) according to the manufacturer's instructions. Briefly, 5 µl of lysate was mixed with 200 µl of working solution of kit and incubated for 3 min. Absorbance was measured at 612 nm using a microplate reader.
Statistical analysis
Statistical data analysis was performed by one-way analysis of variance (ANOVA) on n = 3 experiments. Statistical significance was assigned as *P < 0.05, **P < 0.01.
Supplemental data
Supplemental data include five figures, two tables and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-43-6-06.pdf.
Acknowledgements
This work was supported by a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean Government (MEST) (No. 2010-0018294) and WCU (World Class University) program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (Project No. R31-2008-000-10105-0). We would like to thank Dr. Jung Min Hong for technical assistance.
Abbreviations
- ALP
alkaline phosphate
- C
cyclic strain
- CN
control
- COL-I
collagen type I (COL1A1)
- OC
osteocalcin (BGLAP)
- OSX
osterix (SP7)
- RUNX2
runt-related transcription factor 2 (CBFA1)
- U
ultrasound stimulation
- U+C
combined stimulation
Supplementary Material
Supplemental Data
References
- 1.Ali MM, Yoshizawa T, Ishibashi O, Matsuda A, Shimomura J, Mera H, Nakashima K, Kawashima H. PIASx beta is a key regulator of osterix transcriptional activity and matrix mineralization in osteoblasts. J Cell Sci. 2007;120:2565–2573. doi: 10.1242/jcs.005090. [DOI] [PubMed] [Google Scholar]
- 2.Corradi C, Cozzolino A. The action of ultrasound on the evolution of an experimental fracture in rabbits. Minerva Ortop. 1952;66:77–98. [Google Scholar]
- 3.Davisson T, Kunig S, Chen A, Sah R, Ratcliffe A. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J Orthop Res. 2002;20:842–848. doi: 10.1016/S0736-0266(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 4.Griffith LG, Naughton G. Tissue engineering-Current challenges and expanding opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 5.Hedrick WR, Hykes DL, Starchman DE. Ultrasound physics and instrumentation. St. Louis, MO: Mosby Year Book; 1995. [Google Scholar]
- 6.Kokubu T, Matsui N, Fujioka H, Tsunoda M, Mizuni K. Low intensity pulsed ultrasound exposure increases prostaglandin E2 production via the induction of cyclooxygenase-2 mRNA in mouse osteoblast. Biochem Biophys Res Commun. 1999;256:284–287. doi: 10.1006/bbrc.1999.0318. [DOI] [PubMed] [Google Scholar]
- 7.Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36:1047–1055. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Lan PX, Lee JW, Seol YJ, Cho DW. Development of 3D PPF/DEF scaffolds using micro-stereolithography and surface modification. J Mater Sci-Mater Med. 2009;20:271–279. doi: 10.1007/s10856-008-3567-2. [DOI] [PubMed] [Google Scholar]
- 9.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 10.Lee AJ, Hodges S, Eastell R. Measurement of osteocalcin. Ann Clin Biochem. 2000;37:432–446. doi: 10.1177/000456320003700402. [DOI] [PubMed] [Google Scholar]
- 11.Lee HL, Yi TG, Woo KM, Ryoo HM, Kim GS, Baek JH. Msx2 mediates the inhibitory action of TNF-α on osteoblast differentiation. Exp Mol Med. 2010;42:437–445. doi: 10.3858/emm.2010.42.6.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SJ, Kang HW, Park JK, Rhie JW, Hahn SK, Cho DW. Application of microstereolithography in the development of three-dimensional cartilage regeneration scaffolds. Biomed Microdevices. 2008;10:233–241. doi: 10.1007/s10544-007-9129-4. [DOI] [PubMed] [Google Scholar]
- 13.Lu L, Peter SJ, Lyman MD, Lai HL, Leite SM, Tamada JA, Vacanti JP, Langer R, Mikos A. In vitro degradation of porous poly(L-lactic acid) foams. Biomaterials. 2000;21:1595–1605. doi: 10.1016/s0142-9612(00)00048-x. [DOI] [PubMed] [Google Scholar]
- 14.Mauney JR, Sjostorm S, Blumberg J, Horan R, O'Leary JP, Vunjak-Novakovic G, Volloch V, Kaplan DL. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int. 2004;74:458–468. doi: 10.1007/s00223-003-0104-7. [DOI] [PubMed] [Google Scholar]
- 15.Meyer U, Buchter A, Nazer N, Wisemann HP. Design and performance of a bioreactor system for mechanically promoted three-dimensional tissue engineering. Br J Oral Maxillofac Surg. 2006;44:134–140. doi: 10.1016/j.bjoms.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: Reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of bone extracellular matrix. J Cell Physiol. 1990;143:420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 17.Park IB, Ha YM, Kim MS, Lee SH. Fabrication of a micro-lens array with a non-layerd method in projection microstereolithography. Int J Precis Eng Manuf. 2010;11:483–490. [Google Scholar]
- 18.Rath B, Nam J, Knobloch TJ, Lannutti JJ, Agarwal S. Compressive forces induce osteogenic gene expression in calvarial osteoblasts. J Biomech. 2008;41:1095–1103. doi: 10.1016/j.jbiomech.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reher P, Doan N, Bradnock B, Meghji S, Harris M. Therapeutic ultrasound for osteoradionecrosis: An in vitro comparison between 1 MHz and 45 kHz machines. Eur J Cancer. 1998;34:1962–1968. doi: 10.1016/s0959-8049(98)00238-x. [DOI] [PubMed] [Google Scholar]
- 20.Rubin C, Bolander M, Ryaby JP, Hadjiargyrou M. The use of low-intensity ultrasound to accelerate the healing of fractures. J Bone Joint Surg Am. 2001;83-A:259–270. doi: 10.2106/00004623-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Saito M, Fujii K, Tanaka T, Sochi S. Effect of low- and high-intensity pulsed ultrasound on collagen post-translational modifications in MC3T3-E1 osteoblasts. Calcif Tissue Int. 2004;75:384–395. doi: 10.1007/s00223-004-0292-9. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Asou Y, Sekiya I, Sotome S, Orii H, Shinomiya K. Enhancement of tissue engineered bone formation by a low pressure system improving cell seeding and medium perfusion into a porous scaffold. Biomaterials. 2006;27:2738–2746. doi: 10.1016/j.biomaterials.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Yang RS, Lin WL, Chen YZ, Tang CH, Huang TH, Lu BY, Fu WM. Regulation by ultrasound treatment on the integrin expression and differentiation of osteoblasts. Bone. 2005;36:276–283. doi: 10.1016/j.bone.2004.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data