Table 2.
Cu-Catalyzed Desulfitative Coupling using a “Metallothionein Mimic”
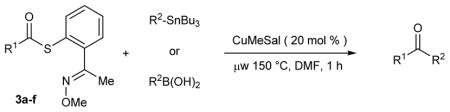 | |||
|---|---|---|---|
| entry | R1 | R2 | ketone (%) |
| Boronic Acid Couplings using 1.2 equiv of R2B(OH)2a | |||
| 1 | p-tolyl | 4-chlorophenyl | 91 |
| 2 | p-tolyl | 4-cyanophenyl | 88 |
| 3 | p-tolyl | 4-(methoxycarbonyl)phenyl | 86 |
| 4 | p-tolyl | 4-(formyl)phenyl | 80 |
| 5 | p-tolyl | 3-acetylphenyl | 71 |
| 6 | p-tolyl | 3-formylphenyl | 69 |
| 7 | p-tolyl | 3-hydroxyphenyl | 70 |
| 8 | p-tolyl | 2,5-dimethoxyphenyl | 68 |
| 9 | p-tolyl | trans-2-(4-chlorophenyl)vinyl | 52 |
| 10 | n-propyl | 4-(methoxycarbonyl)phenyl | 82 |
| 11 | CH2OAc | 3-furyl | 73 |
| 12 | CH2OAc | 4-(methoxycarbonyl)phenyl | 78 |
| 13 | (E)-1-propenyl | 4-methoxyphenyl | 80 |
|
| |||
| Organostannane Couplings using 1.1 equiv of R2SnBu3b | |||
| 14 | p-tolyl | 4-methoxyphenyl | 95 |
| 15 | p-tolyl | 4-fluoro-3-methylphenyl | 81 |
| 16 | p-tolyl | 4-iodophenyl | 68 |
| 17 | thienyl | 4-methoxyphenyl | 86 |
| 18 | n-propyl | 2-methoxypyridin-2-yl | 70 |
| 19 | Cyclohexyl | 4-methoxyphenyl | 62 |
Typical experimental procedure: A dry microwave tube (10 mL) was equipped with a magnetic stir bar. To the tube was added the corresponding thiol ester (0.1 mmol), boronic acid (0.12 mmol) and CuMeSal (0.02 mmol). The reaction tube was flushed with argon and sealed. Through the septum anhydrous and degassed DMF (1 mL) was added. The mixture was subsequently heated in a microwave reactor at 150 °C for 1 h. After cooling, ethyl ether (10 mL) was added to the mixture. The reaction mixture was washed with water (2×5 mL), brine (5 mL), dried over MgSO4 and evaporated. The residue was purified by preparative plate silica chromatography using hexanes/EtOAc as the eluent.
Typical experimental procedure: A dry microwave tube (10 mL) was equipped with a magnetic stir bar. To the tube were added the corresponding thiol ester (0.1 mmol) and CuMeSal (0.02 mmol). The reaction vessel was flushed with argon and sealed. Through the septum the organostanane (0.11 mmol) dissolved in anhydrous and degassed DMF (1 mL) was added. The mixture was subsequently heated in a microwave reactor at 150 °C for 1 h. After cooling, ethyl ether (10 mL) was added to the mixture. The reaction mixture was washed with water, brine, dried over MgSO4 and evaporated. The residue was purified by preparative plate silica chromatography using hexanes/EtOAc as the eluent.
