Abstract
BACKGROUND:
A single nucleotide variation within catechol-o-methyl transferase (COMT) gene may alter the COMT enzyme activity level. Polymorphism of Val158Met in the COMT gene has been related to malignancy. In this regard, a study was carried out to find a possible association between the COMT gene polymorphism in patients with sporadic prostate cancer (PCa) and benign prostatic hyperplasia (BPH).
METHODS:
All types of COMT158 Val/Met polymorphism were carried out using ASO-PCR method in 41 patients with prostate cancer, 193 patients with benign prostatic hyperplasia and 107 healthy male individuals.
RESULTS:
The results of this study showed that the frequency of low producer allele A at codon 158 of the COMT gene is significantly different in BPH group compared to normal male control group (OR, 95% CI, p value 1.95: 1.46, 2.44, 0.021, respectively). However no significant difference was noticed when the comparison was made between prostate cancer group and normal male control group and also between BPH and PCa groups.
CONCLUSIONS:
Decreased level of catechol-o-methyl transferase gene activity may play a possible role in benign prostatic hyperplasia development but not in prostate cancer. Increased level of COMT gene activity has a protective role against BPH.
Keywords: Prostate Cancer, Benign Prostatic Hyperplasia, COMT 158Val/Met Polymorphism
Prostate cancer (PCa) is the most common serious complication among men in Europe and North America.1 The distribution of prostate cancer is significantly different in different countries.2,3 Habibi et al reported that prostate cancer is not common in Iranian population.4 It has been shown in some studies that single nucleotide polymorphisms within vitamin D receptor, androgen receptor and catechol-o-methyltransferase (COMT) genes have been associated with benign prostatic hyperplasia and prostate cancer.5–7 The COMT gene is located on chromosome 22, band q11.2 and encodes S-COMT (soluble COMT) and MB-COMT (membrane-bound COMT) proteins.7,8 Both proteins are produced in most of human tissues. A 1.5 kb mRNA is produced in human brain.9 The S-COMT expression is high in most human tissues, but in human brain, S-COMT expression level is 30% of total transcripts and the rest belongs to MB-COMT expression.10 Tissue specific transcription factors have a role in variable regulation of human COMT gene expression.11 In the human tissues, three phenotypes of the COMT enzyme activities including COMT A/A (enzyme with low activity), COMT A/G (enzyme with medium activity), and COMT G/G (enzyme with high activity) have been seen.12 The COMT gene has several polymorphisms. Three polymorphic sites are at codons 62, 72 and 158. The codon 158 is localized in exon 4.13,14 In COMT gene, a transition of G→A at codon 158 leads to changes in the protein function as three to four times decrease in the level of enzyme activity.15 The influence of COMT G158A polymorphism has been studied in some human cancers such as breast,16–19 endometrial20 and prostate carcinomas.21 The estrogen present in the cells may undergo hydroxylation procedure and the resultant catechol estrogen may cause DNA single-strand breakage and mutation. These catechol estrogens may play a causative role in the carcinogenesis process and may affect the prostate.22 COMT enzyme is one of the major enzymes that attaches these highly reactive and carcinogenic catechol estrogens and detoxifies them. In this way, the COMT enzyme has a protective role and the presence of different polymorphisms in the COMT gene could affect the degree of the protectiveness of its produced enzyme. Since genetic variations are inheritable factors and their frequency differs in different populations with varied ethnicity and background, the present study was designed to investigate the polymorphisms of the COMT gene in an Iranian population to determine their probable association with BPH and sporadic prostate cancer risk.
Methods
The samples were collected during the period of 2005 to 2007. Forty one patients with sporadic prostate carcinoma, 193 patients with BPH, and 107 normal males entered the study. Prostate cancer and BPH samples were obtained from the patients hospitalized at the department of urology in the educational university hospital of Imam Khomeini in Urmia, Iran. Control group were selected from old men who came to the same hospital for regular ophthalmologic check up. To make sure that selected controls were healthy and free of cancer, a history was taken from the patients and they underwent physical examination by a physician for detecting any possible clinical symptoms and signs of BPH or PCa. Moreover, their serum levels of prostate-specific antigen (PSA) were measured. Measurement of PSA was also carried out for patients of BPH and PCa groups at the time of diagnosis. The diagnosis of BPH or PCa in these two groups was confirmed by pathologic studies of prostate specimens. To study the polymorphisms of the COMT gene, five milliliters of peripheral blood was collected in EDTA containing tube. Genomic DNA was extracted using salting out method.23 Allele specific oligonucleotides-polymerase chain reaction (ASO-PCR) was carried out for detection of COMT 158Val/Met polymorphism using the primers as described by Tanaka et al (Table 1).7 Each PCR reaction was performed on 25 μl containing 50-100 ng DNA, 10 pmol of each primer, 175 μmol dNTPs, 1.5 mM MgCl2, 1x PCR buffer, and 0.3 U Taq DNA polymerase enzyme (Cinnagen, Tehran, Iran). Thermocycling program (Eppendorf mastercycler, Germany) consisted of initial denaturation at 96°C 10 min; 30 cycles: 96°C for 30 s, 56°C for 45 s, 72°C for 45 s; and final extension at 72°C for 5 min. Presence or absence of each allele (PCR products) were observed by electrophoresis on 2% agarose gel. After electrophoresis, amplified products were visualized by ethidium bromide stain using ultraviolet trans-illumination (Figure 1).
Table 1.
Sequences of the used primers

Figure 1.
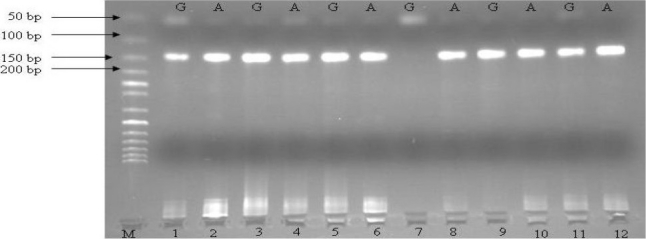
Six BPH patients checked for COMT gene polymorphism. For each patient, two reactions were set up. Patient genotype is AA, AG, or GG. M: 100 bp DNA Ladder in Lane 1
Statistical Analysis
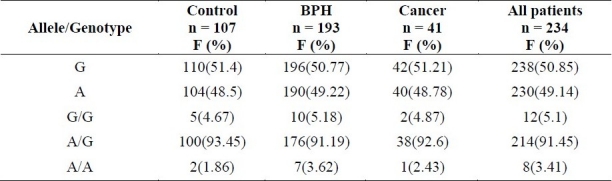
Genotypic and allelic frequencies of the studied COMT polymorphisms at codon 158 in the controls, BPH, and prostate cancer patients are shown in table 2. Genotype frequencies of COMT polymorphisms at codon 158 were compared using χ2 test. P value level less than 0.05 was considered significant. The minimum calculated sample size for each group was 38 (two sided test, power (1-â): 85% level of significance á: 5%). Therefore, having 41 cases of prostate carcinoma is acceptable. The SPSS version 16.0 for windows and Microsoft Excel 2007 were used to calculate the Pearson χ2, the odds ratio, 95% confidence interval (lower-upper) and other data processing.
Table 2.
The genotypic and allelic frequencies of COMT gene 158 polymorphism in healthy control, BPH and prostate cancer groups
Results
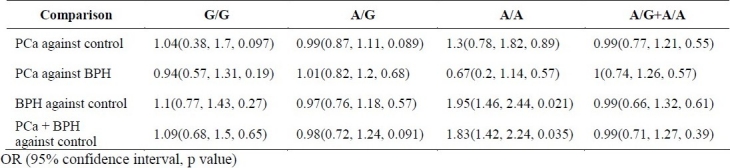
The mean age was 74 ± 10 years in PCa group, 70.6 ± 10 years in BPH group, and 71.2 ± 10 years in control group. Mean PSA level in BPH and PCa group were 3.47 ± 2.8 and 19.12 ± 17 ng/ml, respectively. Mean prostate volume on ultrasound examination was 68.8 ± 36 and 58.9 ± 28.3 cc in PCa and BPH groups, respectively. All polymorphic frequencies for healthy controls followed the Hardy-Weinberg equilibrium. Among PCa patients, no differences in genotype or allele frequencies were detected when compared with controls for the COMT G158A polymorphism (All p values were more than 0.05 as mentioned in table 3). However, risks for BPH were significant for the variant genotype and allele at this site. The variant allele A proved to be a risk for BPH (P = 0.021) (Table 3). When comparison of both BPH and PCa groups was done with normal group, the difference was statistically significant too (P = 0.035).
Table 3.
Odd ratio (OR), Confidence interval, and p value of three compared healthy control, BPH and prostate cancer groups
Discussion
It has been shown that activated estrogens via CYP1A1 or CYP1B1 24 are associated with several tumors in various tissues, including prostate.25 One of the resultant chemical structures responsible for the tumorigenicity of CYPs is catechol estrogens. This form of estrogen leads to the formation of semi-quinones and quinones which are known to react with DNA.7,26 Therefore, detoxification of catechol estrogens in the cells of the body is important to prevent mutations, and the COMT enzyme is responsible for this defense. Proper enzymatic function of COMT in cells is thus essential to prevent genetic changes. Polymorphisms of genes, however, have been shown to alter enzymatic activity. In the case of CYP2A6, a polymorphism results in reduced activity of the enzyme.27 Polymorphism is a potential mechanism by which COMT activity is reduced, thereby causing an accumulation of mutagenic catechol compounds. The polymorphism at codon 158 of the COMT gene results in amino acid substitutions as Val→Met. In the present study, this polymorphic site was analyzed to determine whether the variant polymorphism influences the risk of prostatic disease. Significant difference was observed in genotypic or allelic frequency of this site between BPH and normal healthy controls. However our results did not find any association between Met158 polymorphism and prostate cancer. These results for Met158 are different from those obtained from the study done on prostate cancer in Japan by Suzuki et al.21 They showed that G/A genotype of the COMT gene is associated with a weak tendency toward increased prostate carcinoma risk.21
However the population of their study was familial prostate carcinoma patients whose first-degree relatives had prostate carcinoma, while we conducted our study on patients with sporadic prostate carcinoma. Tanaka et al showed that COMT Met158 polymorphism is a risk factor for prostate cancer but not BPH in comparison with normal controls.7 The differences between the results of Tanaka et al study and our study may be due to the sample size, as the present study included only 41 patients with prostate cancer while they had 178 patients with PCa. Perhaps, a greater sample size of prostate carcinoma might have more defensible results. For BPH group on the other hand, we had a greater sample size (193) compared to Tanaka et al study (134).7 In this regard, we observed that when comparison was made between both BPH and PCa as a single group with control group, the difference was statistically meaningful (P = 0.035). However, as Habibi et al have reported, the rate of prostate cancer is very low in Iran 4 and in this way reaching to an acceptable number of cases of prostate carcinoma to achieve valid results may be a difficult task.
Conclusions
Decreased level of COMT enzyme activity may play a possible role in BPH development. Increased level of COMT enzyme activity has a protective effect against BPH.
Authors’ Contributions
MDO was supervisor and designer of the project. SB was a resident in urology department and along with HY, another resident, collected the cases and participated in clinical evaluation of the patients. MB is our MCs staff and helped us in all practical part of the project such as DNA extraction, PCR process and so on.
All Authors have read and approved the content of final manuscript.
Acknowledgments
This study was supported by a research grant from the research dean of Urmia University of Medical Sciences.
Footnotes
Conflict of Interests
Authors have no conflict of interests regarding this article.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Gronberg H. Prostate cancer epidemiology. The Lancet. 2003;361(9360):859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 3.Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97(1):72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 4.Habibi A, Manouchehri A, Diba MH, Sajadi M, Ghavam M. Prostatic tumors in Iran. Int Surg. 1975;60(8):405–7. [PubMed] [Google Scholar]
- 5.Habuchi T, Suzuki T, Sasaki R, Wang L, Sato K, Satoh S, et al. Association of vitamin D receptor gene polymorphism with prostate cancer and benign prostatic hyperplasia in a Japanese population. Cancer Res. 2000;60(2):305–8. [PubMed] [Google Scholar]
- 6.Stanford JL, Just JJ, Gibbs M, Wicklund KG, Neal CL, Blumenstein BA, et al. Polymorphic repeats in the androgen receptor gene: molecular markers of prostate cancer risk. Cancer Res. 1997;57(6):1194–8. [PubMed] [Google Scholar]
- 7.Tanaka Y, Sasaki M, Shiina H, Tokizane T, Deguchi M, Hirata H, et al. Catechol-O-methyltransferase gene polymorphisms in benign prostatic hyperplasia and sporadic prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(2):238–44. doi: 10.1158/1055-9965.EPI-05-0550. [DOI] [PubMed] [Google Scholar]
- 8.Salminen M, Lundstrom K, Tilgmann C, Savolainen R, Kalkkinen N, Ulmanen I. Molecular cloning and characterization of rat liver catechol-o-methyltransferase. Gene. 1990;93(2):241–7. doi: 10.1016/0378-1119(90)90231-f. [DOI] [PubMed] [Google Scholar]
- 9.Hong J, Shu-Leong H, Tao X, Lap-Ping Y. Distribution of catechol-O-methyltransferase expression in human central nervous system. Neuroreport. 1998;9(12):2861–4. doi: 10.1097/00001756-199808240-00033. [DOI] [PubMed] [Google Scholar]
- 10.Tenhunen J, Ulmanen I. Production of rat soluble and membrane-bound catechol O-methyltransferase forms from bifunctional mRNAs. Biochem J. 1993;296(Pt 3):595–600. doi: 10.1042/bj2960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeanjean AP, Laterre EC, Maloteaux JM. Neuroleptic binding to sigma receptors: possible involvement in neuroleptic-induced acute dystonia. Biol Psychiatry. 1997;41(10):1010–9. doi: 10.1016/s0006-3223(96)00264-8. [DOI] [PubMed] [Google Scholar]
- 12.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Saito S, Iida A, Sekine A, Miura Y, Sakamoto T, Ogawa C, et al. Identification of 197 genetic variations in six human methyltransferase genes in the Japanese population. J Hum Genet. 2001;46(9):529–37. doi: 10.1007/s100380170035. [DOI] [PubMed] [Google Scholar]
- 14.Boudikova B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol-O-methyltransferase pharmacogenetics. Clin Pharmacol Ther. 1990;48(4):381–9. doi: 10.1038/clpt.1990.166. [DOI] [PubMed] [Google Scholar]
- 15.Miyoshi Y, Noguchi S. Polymorphisms of estrogen synthesizing and metabolizing genes and breast cancer risk in Japanese women. Biomed Pharmacother. 2003;57(10):471–81. doi: 10.1016/j.biopha.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Wedrén S, Rudqvist TR, Granath F, Weiderpass E, Ingelman-Sundberg M, Persson I, et al. Catechol-O-methyltransferase gene polymorphism and post-menopausal breast cancer risk. Carcinogenesis. 2003;24(4):681–7. doi: 10.1093/carcin/bgg022. [DOI] [PubMed] [Google Scholar]
- 17.Sazci A, Ergul E, Utkan NZ, Canturk NZ, Kaya G. Catechol-O-methyltransferase Val 108/158 Met polymorphism in premenopausal breast cancer patients. Toxicology. 2004;204(2-3):197–202. doi: 10.1016/j.tox.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 18.Wen W, Cai Q, Shu XO, Cheng JR, Parl F, Pierce L, et al. Cytochrome P450 1B1 and catechol-O-methyltransferase genetic polymorphisms and breast cancer risk in Chinese women: results from the Shanghai breast cancer study and a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(2):329–35. doi: 10.1158/1055-9965.EPI-04-0392. [DOI] [PubMed] [Google Scholar]
- 19.McGrath M, Hankinson SE, Arbeitman L, Colditz GA, Hunter DJ, De Vivo I. Cytochrome P450 1B1 and catechol-O-methyltransferase polymorphisms and endometrial cancer susceptibility. Carcinogenesis. 2004;25(4):559–65. doi: 10.1093/carcin/bgh039. [DOI] [PubMed] [Google Scholar]
- 20.Doherty JA, Weiss NS, Freeman RJ, Dightman DA, Thornton PJ, Houck JR, et al. Genetic factors in catechol estrogen metabolism in relation to the risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(2):357–66. doi: 10.1158/1055-9965.EPI-04-0479. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki K, Nakazato H, Matsui H, Koike H, Okugi H, Kashiwagi B, et al. Genetic polymorphisms of estrogen receptor alpha, CYP19, catechol-O-methyltransferase are associated with familial prostate carcinoma risk in a Japanese population. Cancer. 2003;98(7):1411–6. doi: 10.1002/cncr.11639. [DOI] [PubMed] [Google Scholar]
- 22.Kunugi H, Nanko S, Ueki A, Otsuka E, Hattori M, Hoda F, et al. High and low activity alleles of catechol-O-methyltransferase gene: ethnic difference and possible association with Parkinson's disease. Neurosci Lett. 1997;221(2-3):202–4. doi: 10.1016/s0304-3940(96)13289-4. [DOI] [PubMed] [Google Scholar]
- 23.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams JA, Martin FL, Muir GH, Hewer A, Grover PL, Phillips DH. Metabolic activation of carcinogens and expression of various cytochromes P450 in human prostate tissue. Carcinogenesis. 2000;21(9):1683–9. doi: 10.1093/carcin/21.9.1683. [DOI] [PubMed] [Google Scholar]
- 25.Cavalieri EL, Devanesan P, Bosland MC, Badawi AF, Rogan EG. Catechol estrogen metabolites and conjugates in different regions of the prostate of Noble rats treated with 4-hydroxyestradiol: implications for estrogen-induced initiation of prostate cancer. Carcinogenesis. 2002;23(2):329–33. doi: 10.1093/carcin/23.2.329. [DOI] [PubMed] [Google Scholar]
- 26.Thibodeau PA, Paquette B. DNA damage induced by catecholestrogens in the presence of copper (II): generation of reactive oxygen species and enhancement by NADH. Free Radic Biol Med. 1999;27(11-12):1367–77. doi: 10.1016/s0891-5849(99)00183-5. [DOI] [PubMed] [Google Scholar]
- 27.Kitagawa K, Kunugita N, Kitagawa M, Kawamoto T. CYP2A6*6, a novel polymorphism in cytochrome p450 2A6, has a single amino acid substitution (R128Q) that inactivates enzymatic activity. J Biol Chem. 2001;276(21):17830–5. doi: 10.1074/jbc.M009432200. [DOI] [PubMed] [Google Scholar]


