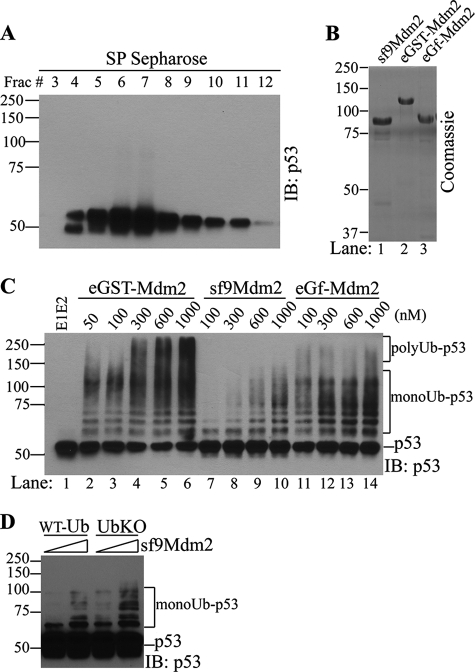
FIGURE 1.
Mdm2 only mediates multiple monoubiquitination of p53 in vitro. A, recombinant p53 used in the in vitro reaction was purified from E. coli by His tag affinity purification followed by SP Sepharose cation-exchange chromatography. p53 Western blots of SP Sepharose fractions (Frac) are shown. A pool of p53 (fractions 8–11) was used as substrates for in vitro assay. B, Coomassie staining of Mdm2 recombinant proteins used in the in vitro assay. sf9Mdm2, HA-Mdm2-His purified from sf9 cells; eGST-Mdm2, GST-Mdm2 purified from E. coli; eGf-Mdm2, GST-free E. coli expressed Mdm2. C and D, in vitro p53 ubiquitination by GST-Mdm2 or GST-free Mdm2 at concentrations indicated. Ubiquitinated p53 was detected by Western blot analysis of p53 after the reaction using different versions of Mdm2 (C) or ubiquitin (D) as indicated. IB, immunoblot; WT-Ub, wild type ubiquitin; UBK0, lysine-less ubiquitin. polyUb-p53, polyubiquitinated p53 (p53-ubiquitin adducts >140 kDa); monoUb-p53, multimonoubiquitinated p53 (seven bands above p53).