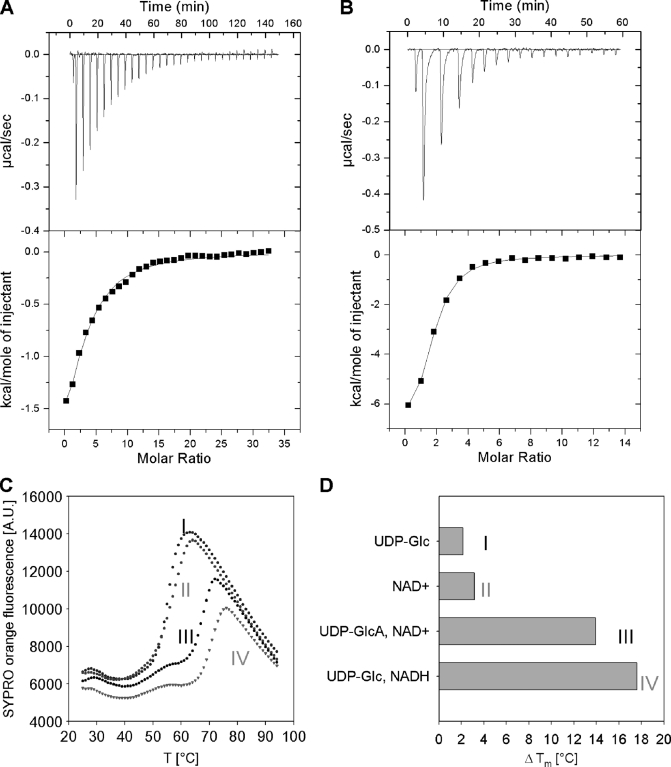
FIGURE 5.
Ligand binding to hUGDH measured by ITC (A and B) and differential scanning fluorometry (C and D). ITC data for binding of UDP-Glc (1 mm) to wild-type hUGDH (33 μm) (A) and binding of NAD+ (0.5 mm) to a complex of a C276A mutant (22 μm) and UDP-Glc (1 mm) (B). C, differential scanning fluorometry with hUGDH (1 μm) in the presence of 1 mm each UDP-Glc (I), NAD+ (II), UDP-GlcUA and NAD+ (III), as well as UDP-Glc and NADH (IV). SYPRO Orange fluorescence is used as reporter of enzyme denaturation. D, difference (ΔTm) in apparent melting temperature (Tm) for enzyme incubated in the presence and absence of the added compounds. The Tm of hUGDH in buffer is 50 °C. The Tm was calculated by fitting a Boltzmann sigmoid to the data. Measurements were done in triplicate, and standard errors on all parameters were ≤15%.