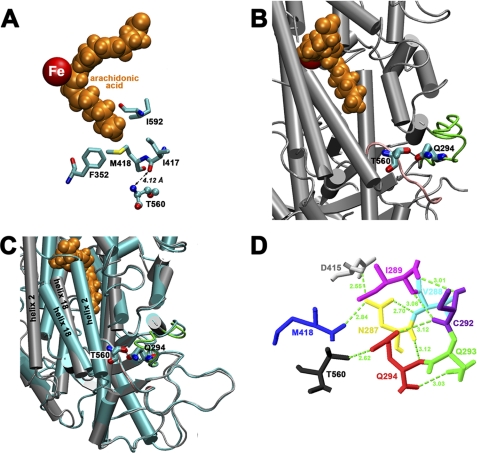
FIGURE 1.
Localization of Thr-560 in the three-dimensional structure of ALOX15 and its involvement in a network of hydrogen bonds. A, Thr-560 is not an immediate active site residue. Although it is located in proximity to Ile-417, there is no direct interaction to any of the triade constituents (Phe-352, Ile-417, Met-418, and Ile-592; numbering according to human ALOX15). The shortest distance (4.12 Å) was measured between the backbone carbonyl of Ile-417 and the backbone nitrogen of Thr-560, which is beyond the binding distance of noncovalent interactions. B, the side chain OH group of Thr-560 hydrogen bridges with side chain oxygen of Gln-294. This hydrogen bridge might contribute to stabilize the less well structured loop regions indicated in green and pink. C, the loop regions surrounding Thr-560 and Gln-294 do not undergo major structural rearrangement upon ligand binding at the active site. In fact, the bonding distance of the Thr-560–Gln-294 hydrogen bridge, which is 2.62 Å in the ligand-free structure, only increases to 2.72 Å in the ligand-bound form. As indicated before, there is a pronounced relocation of helix 2 during ligand binding. Gray, ligand-free conformer; light blue, ligand-bound conformer; orange, arachidonic acid. D, Gln-294 is a key residue in a hydrogen bond network, which connects the side chain of Thr-560 with Met-418, a member of the amino acid triade that determines the positional specificity of mammalian 12/15-LOXs. The images were prepared with the VMD software package (26). Hydrogen bridges were determined with Swiss-PdbViewer, v4.0.1.