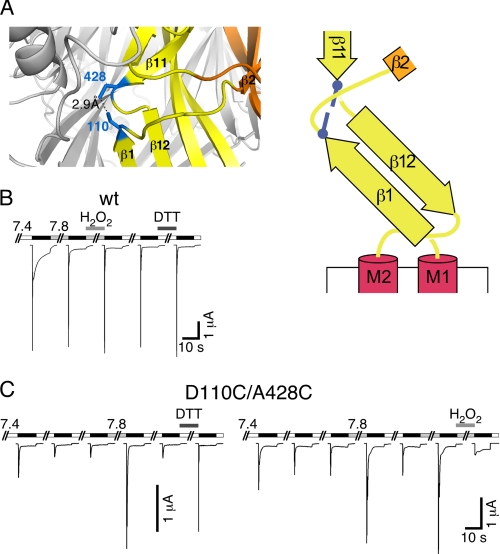
FIGURE 9.
Cross-linking of residues 110 and 428 traps sASIC1b in the desensitized state. A, left panel, detail from the cASIC1 crystal structure, in which two Cys residues had been modeled: one at position 82 and one at position 413 (corresponding to positions 110 and 428 of sASIC1b). Right panel, schematic representation with the two cysteines as blue bars. B, reducing and oxidizing conditions had no effect on sASIC1b-wt currents. C, left panel, sASIC1b-D110C/A428C current amplitude gradually decreased with repeated stimulation by ligand (pH 5.0). Switching the pH to 7.8 or reducing conditions strongly increased current amplitude. Right panel, oxidizing conditions strongly reduced current amplitude of sASIC1b-D110C/A428C.