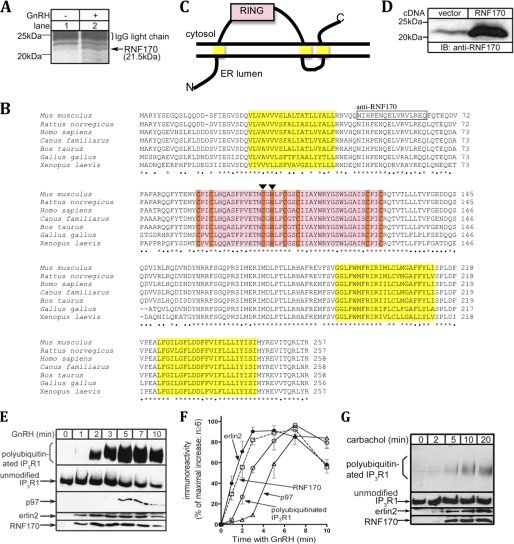
FIGURE 1.
RNF170 is a RING domain-containing protein that associates with activated IP3 receptors. A, αT3-1 cells were incubated without or with 100 nm GnRH for 7 min and harvested with 1% CHAPS lysis buffer, and then anti-IP3R1 immunoprecipitates were subjected to SDS-PAGE and silver staining. The 20- to 25-kDa region of a representative gel is shown, and the 21.5-kDa band marked with an arrow was identified as RNF170. B, a multiple-sequence alignment of RNF170 homologs from selected vertebrates using ClustalW. Amino acid identity among all species shown is indicated with asterisks, whereas identity between at least rodents and humans is indicated with dots. Predicted are three putative transmembrane domains (yellow, TMHMM Server v 2.0), a RING-HC domain (pink, with zinc-coordinating residues in orange (9)), ER membrane localization (PSORTb v 3.0), and a molecular weight of ∼30 kDa (ExPASy Compute pI/MW). The peptide sequence boxed was used to raise anti-RNF170, and the individual residues marked with arrowheads were mutated in *RNF170FLAG. C, topological model of RNF170 based on predictions of three transmembrane domains and the RING domain facing the cytosol. D, HeLa cells were transfected with either vector (lane 1) or with a construct containing the RNF170 encoding sequence isolated from αT3-1 cells (lane 2), and RNF170 expression was assessed in immunoblots (IB) with anti-RNF170. E, αT3-1 cells were treated for the indicated times with 100 nm GnRH and harvested with 1% CHAPS lysis buffer, and then anti-IP3R1 immunoprecipitates were probed for ubiquitin, IP3R1, p97, erlin2, and RNF170. F, the kinetics of GnRH-induced IP3R1 ubiquitination and coimmunoprecipitation of p97, erlin2, and RNF170 were quantitated and graphed. G, SH-SY5Y cells were treated for the indicated times with 1 mm carbachol and harvested with 1% CHAPS lysis buffer, and then anti-IP3R1 immunoprecipitates were probed for ubiquitin, IP3R1, erlin2, and RNF170. In E and G, polyubiquitinated IP3R1, unmodified IP3R1, p97, erlin2, and RNF170 migrated at 275–380 kDa, 260 kDa, 97 kDa, 43 kDa, and 21.5 kDa, respectively.