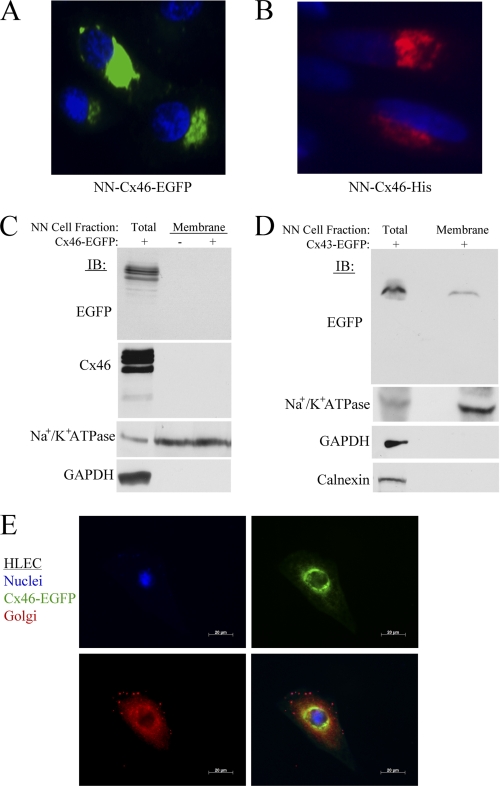
FIGURE 9.
Cx46 protein is predominantly localized to the intercellular compartments in lens NN1003A cells. A, fluorescence microscopy images of NN1003A cells stably overexpressing Cx46-EGFP. All cells were fixed and stained with DAPI to visualize nuclei. B, the localization of Cx46-His protein (pentahistidine-tagged) in NN1003A cells. Cells were transfected with plasmid encoding Cx46-His and, after 24 h of transfection, cells were fixed, permeabilized, and labeled with Penta-His antibody (red) and DAPI (blue). C, Western blot analyses were performed for Cx46 (with anti-EGFP or anti-Cx46) on equal amounts of total protein from lysates of Cx46-EGFP-expressing cells (lane 1) and plasma membrane protein fractions isolated from untransfected control cells (lane 2) or Cx46-EGFP stable (lane 3) cells. The blots were also probed with antibodies against a plasma membrane marker Na+/K+ ATPase, and a cytosolic marker (GAPDH) to demonstrate the purity of plasma membrane protein extracts. D, Western blot analyses show the localization of Cx43-EGFP in the plasma membrane of Cx43-EGFP-expressing NN1003A cells. Cells were transiently transfected with plasmid encoding Cx43-EGFP for 24 h. Then, the cells were fractionated into plasma membrane protein fractions. Equal amounts of total protein of lysate and plasma membrane fraction of Cx43-EGFP-expressing cells were subjected to Western blot analyses with antibodies against EGFP, Na+/K+ ATPase, GAPDH, and calnexin (an ER marker). E, fluorescence microscopy images of HLEC cells expressing Cx46-EGFP (green) that were fixed, permeabilized, and labeled with 58K/FTCD antibody (red, a Golgi marker) and DAPI (blue). Cx46-EGFP did not co-localize well with the Golgi marker. NN, NN1003A cells.