Abstract
OBJECTIVE
To determine if preimplantation embryos are targets for relaxin secreted from the corpus luteum of the menstrual cycle.
DESIGN
Rhesus monkey oocytes obtained from females undergoing controlled ovarian hyperstimulation were inseminated and the resulting embryos were cultured in medium with or without recombinant human relaxin (20 ng/ml) for 8 days.
SETTING
Research laboratory.
ANIMALS
Rhesus monkey.
INTERVENTIONS
Controlled ovarian stimulation to obtain oocytes for in vitro produced embryos that were cultured with or without human recombinant relaxin.
MAIN OUTCOME MEASURES
The rate of blastocyst development and the percentage of blastocysts and ICM/TE ratio were measured on Day 8 of culture. The presence of relaxin receptor (RXFP1) mRNA in 8 cell embryos was observed by array hybridization.
RESULTS
RXFP1 receptor expression was localized to the inner cell mass of blastocysts as shown by immunohistochemistry. The percentage of embryos that developed to blastocyst and the inner cell mass/ trophectoderm cell ratio was unchanged with relaxin supplementation, however the relaxin-treated embryos developed into blastocysts significantly sooner than untreated embryos.
CONCLUSIONS
These results are the first evidence that the preimplantation primate embryo is a target for relaxin and that the addition of relaxin to in vitro culture medium enhances rhesus monkey embryo development.
Keywords: gene expression, granulosa cells, blastocyst, rhesus macaque
INTRODUCTION
Since first described in the 1920s (1), relaxin has been associated with pregnancy and parturition in many mammalian species (2). In human and nonhuman primates, circulating relaxin is seen during the luteal phase of the menstrual cycle and during early pregnancy, and as early as day 7 post ovulation in humans (3,4) and monkeys (5) It is secreted by human luteinizing granulosa cells as early as day 5 after aspiration during in vitro fertilization cycles (6). Relaxin is also produced by the deciduae and trophoblast (7). While Yki-Jarvinen et al. (8) did not detect relaxin in the oviduct; Tang and Chegini (9) reported relaxin gene expression by RT-PCR with immunohistochemical localization in tubal epithelium of the ampullary and isthmus regions.
Relaxin binds to two receptors that modulate cAMP production, LGR7 and LGR8 (10). These were later renamed RXFP1 and RXFP2 (11), with RXFP1 recognized as the primary receptor for relaxin and RXFP2 as the primary receptor for INSL3 (12). Receptors for relaxin have been localized to a variety of reproductive and non-reproductive tissues by mRNA or protein expression (12).
The roles of relaxin during the pre- and peri-implantation period remain unclear, but relaxin is known to target the endometrium during the peri-implantation period. A peri-implantation effect of relaxin has been known for some time in the mouse (13), but a recent study in the marmoset found that relaxin and uterine RXFP1 were upregulated during this period (14). Relaxin stimulates decidual cell production of IGFBP1, prolactin secretion from decidual and stromal cells, secretion of glycodelin, and VEGF secretion (15, 16). Relaxin may also affect the ovary, which expresses relaxin receptors (17). Another possible target for relaxin would be the embryo itself. The timing of luteal relaxin production indicates it is available to blastocysts and peri-implantation embryo. However, neither the detection of relaxin receptors in blastocysts or peri-implantion embryos nor the effects of H2 relaxin on blastocyst development have been reported. Here we have used the rhesus monkey model to determine if preimplantation embryos may be targets for relaxin secreted during the peri-implantation period.
MATERIALS AND METHODS
Animal husbandry
Adult female rhesus macaques (Macaca mulatta) were housed at the California National Primate Research Center (CNPRC) as described (18). Only females with a history of normal menstrual cycles were selected for this study. All procedures for maintenance and handling of the animals were reviewed and approved in advance by the Institutional Animal Use and Care Administrative Advisory Committee at the University of California at Davis.
Females were observed daily for signs of vaginal bleeding and the first day of menses was assigned cycle day 1. Beginning on cycle day 1–4 recombinant human FSH (r-hFSH: Organon, West Orange, NJ) was administered (37.5 IU) twice daily, intramuscularly for 7 days total. To obtain in vivo matured (VVM) oocytes, females were given recombinant hCG (1000 IU Ovidrel; Serono, Rockland, MA) on treatment day 8 in addition to the FSH treatment outlined above. COCs were removed at 28 – 30 h following hCG by ultrasound-guided aspiration (19, 20). Oocytes were retrieved from aspirates as described (18). Granulosa cells were obtained from the aspirates and immediately placed in buffer and stored frozen for later processing for gene expression.
In vitro fertilization and embryo development
In vivo matured oocytes were rinsed and transferred into TL-PVA medium (37°C) under oil and inseminated according to standard procedure for IVF of rhesus macaque oocytes (20); this is Day 0 of embryo culture. Semen was collected from male macaques that had been trained for this procedure as described (21). Sperm were washed from seminal plasma and resuspended in TL-BSA medium (22). The next morning (Day 1), oocytes were transferred into 70 μl drops of chemically-defined, protein-free hamster embryo culture medium 9 (HECM-9) without or with relaxin (see below) under oil (37°C) and incubated at 37°C in a humidified atmosphere of 5% CO2, 10% O2 and 85% N2 for 48 hours (23). At approximately 60 hours post insemination non-cleaved oocytes were again assessed for developmental status. Non-cleaved oocytes exhibiting a polar body and all embryos were classified as having matured to metaphase II of meiosis (MII). Embryos were transferred into 70 μl drops of HECM-9 medium with 5% bovine calf serum (Gemini Bioproducts, West Sacramento, CA) with or without relaxin (see below) under mineral oil and incubated as described above. Embryos were transferred to fresh medium every other day until fixed on day 8 post insemination. Beginning on Day 5, embryos were observed with a Nikkon SMZ-2B microscope (Melville, NY) housed in a temperature-controlled isolette daily at 0700 and 1700 hrs for developmental stage. On Day 8, the percentage of embryos developing to the blastocyst stage was calculated for each treatment. The % blastocyst development for each female was then averaged to obtain the mean for each treatment.
Blastocyst stage embryos were fixed and stained for differential cell counting with POU5F1 (a.k.a. Oct3/4) as described (18). Optical sectioning was performed with a Delta Vision microscope using a 20x, Olympus UApo 340/0.70 water immersion objective (Olympus Optical Co., Ltd., Tokyo, Japan). A z-projection was created for each blastocyst stage embryo. Samples were coded so that evaluation was performed without knowing sample identity. Manual cell counts were taken of the inner cell mass (ICM) and trophectoderm (TE) cells.
Immunohistochemistry for RXFP1
The mouse monoclonal anti-Lgr7 (AbNova, Walnut, CA) used for immunohistochemistry was previously validated by Western blot producing a 52,000 KD band from lysates of monkey trophoblast cells and human endothelial cells (24). Specificity the antibody has been demonstrated by Western blot analysis of RXFP1 transfected and untransfected HEK293 cells (25). Embryos were fixed in a microtubule-stabilizing buffer for 1 hour and placed in blocking solution overnight at 4°C (26). All embryos were transferred from blocking solution into 30 μl drops of Dulbecco’s Phosphate Buffered Saline with 0.2% Triton X-100 and 0.1% Tween-20 (DPBS/T/T; all chemicals from Sigma-Aldrich, St. Louis, MO) on separate ten well slides (PolySciences Inc., Warrington, PA). Washes were performed as previously described (18). All embryos were washed (x3) with DPBS/T/T. Staining for RXFP1 (Lgr7) was performed by incubating embryos in a humidified chamber overnight at 4°C with mouse monoclonal anti-Lgr7 diluted 1:100 in DPBS/T/T. The primary antibody was substituted with non-immune fetal bovine serum (Gemini Bioproducts, West Sacramento, CA) diluted 1:100 in DPBS/T/T for negative controls. Embryos were washed (x3) with DPBS/T/T and incubated at 37°C for 2 hours in a humidified environment with Alexa Fluor 488 goat anti-mouse IgG (Invitrogen, Carlsbad, CA) diluted 1:100 in DPBS. Embryos were washed in DPBS/T/T (x3), mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, USA) and stored at 4°C in the dark. Embryos were imaged with a DeltaVision microscopy system equipped with a 20x/0.7 water-immersion objective for fluorescence (Applied Precision, Issaquah, WA). The images were analyzed using Adobe Photoshop (Adobe Systems Inc., San Jose, USA). Negative control images were used to determine the appropriate level for thresholding.
Relaxin treatment during in vitro early embryo development
Recombinant human relaxin was obtained from Corthera (San Mateo, CA). A final concentration of 20 ng/ml relaxin was utilized for these studies as this concentration is effective in stimulation of the relaxin receptor (27). To determine the effects of relaxin on early embryo development, mature oocytes were inseminated. The next morning (Day 1) presumptive embryos were placed in fresh HECM-9 drops under oil and cultured with or without relaxin to approximately 60 hours post-insemination (Day 3), when most embryos have reached 8-celled stage of development. On Day 3, cleaved embryos were placed in fresh HECM-9 with 5% BSA medium with or without relaxin and media was changed again on Days 5 and 7.
Statistical Analysis
All data were expressed as the mean ± SEM. Percentage of MII oocytes that cleaved 30 hours after insemination and percentage of blastocyst stage embryos were compared using a 2-tailed paired student’s t-test (n=6 females). Total cell count, ICM cell count, TE cell count and percentage of ICM to TE cells in blastocyst stage embryos were assessed with student’s t tests. Time to blastocyst was assessed with paired t test. Data were analyzed with Prism software (GraphPad Software, Inc., San Diego, USA). Differences were considered statistically significant if P < 0.05.
RESULTS
The relaxin receptor is present in rhesus embryos
The presence of relaxin receptor RXFP1 (a.k.a. LRG7) is shown by immunolabeling with the AbNova Mouse Anti-Lgr7 monoclonal antibody in rhesus monkey blastocyst stage embryos (Figure 1). The panels in Figure 1 are images of reconstructed whole embryos. The labeling of an 8-celled embryo (panels A-C) and two blastocysts (panels D-I) are detailed. The RXFP1 antibody labels the blastomere membranes of the 8-celled embryo (A) as indicated by the punctate labeling that is restricted to the area that is well beyond the nuclear label. As embryo development progresses to the blastocyst stage, the RXFP1 antibody labels only the inner cell mass of the embryo as demonstrated by the intense staining of the ICM (D), but the absence of staining in the trophectoderm cells that are revealed with DAPI nuclear staining (E). The control blastocyst (panels G-H) show the absence of RXFP1 antibody labeling. The merged images (C, F, I) demonstrate the location of RXFP1 labeling relative to the nuclei of the embryos. The RXFP1 mRNA was detected at a low level in a previously published cDNA array study (28) of 8-cell embryos (four arrays, raw intensity value range 31 to 129, average value of 76; data available at www.PREGER.org), further attesting to RXFP1 gene expression in the pre-implantation embryo.
Figure 1.
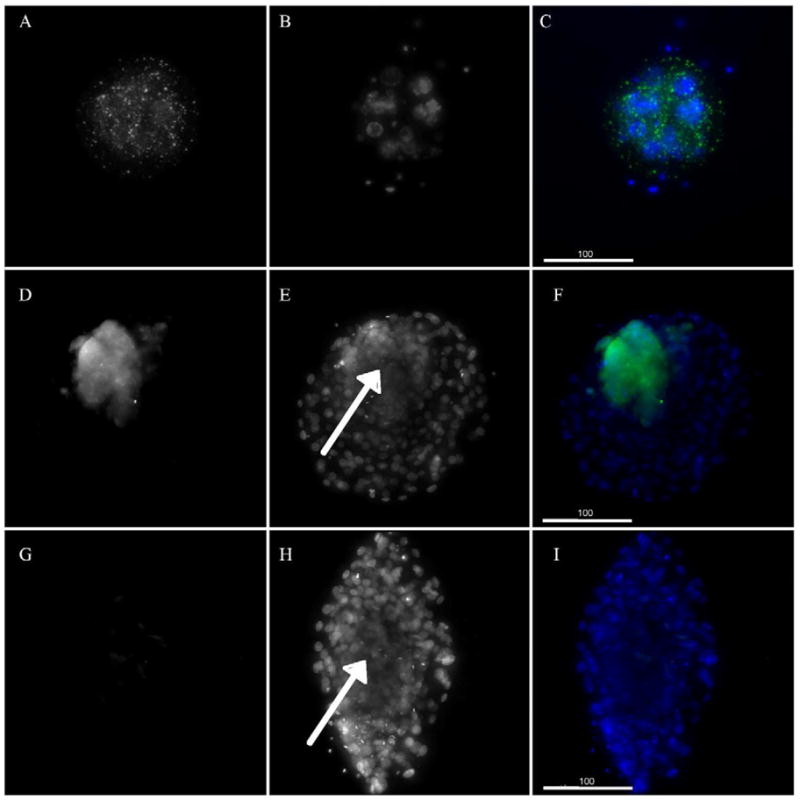
Relaxin receptor antibody staining of monkey 8-celled embryo and blastocysts. Panels A-C show an 8-celled embryo and Panels D-I show blastocysts (Mag 190X), all are whole reconstructed embryos. Bar in lower left corner of panels C,F and I is 100μM Panels A, D and G show the RXFP1 antibody staining. Panel G shows the lack of labeling for the mouse IgG control, Panels B, E and H show DAPI labeling for embryos with arrows in E and H indicating the approximate center of the inner cell mass. Panels C, F and I are merged images of A and B, D and E, and G and H.
Effect of exogenous relaxin on rhesus monkey embryo development in vitro
Having observed RXFP1 mRNA and protein in rhesus embryos, the effect of exogenous relaxin on embryo growth in vitro was investigated. The percentage of embryos reaching the blastocyst stage was similar in the control and relaxin treatment groups (Table 1) and the differential ICM/TE cell counts were similar in both groups of blastocysts (Table 2). However, as shown in Table 1, the time to achieve the blastocyst stage of embryo development was significantly advanced by at least 12 hours for the relaxin group compared to the control group (6.3 ± 0.17 and 7.0 ± 0.13, respectively).
Table 1.
Development of IVF embryos treated with relaxin.
| Treatment Group | # of Embryos cultured | # of Blastocysts | % of Embryos reaching Blastocyst | Days to Blastocyst |
|---|---|---|---|---|
| Control | 56 | 27 | 51.35 ± 11.05 | 7.0 ± 0.13a |
| Relaxin | 70 | 35 | 55.08 ± 8.97 | 6.3 ± 0.17b |
Values are Mean ± SEM
Different superscripts within columns indicates significant (p< 0.05) differences
Table 2.
Differential cell counts of blastocyst stage embryos cultured with or without relaxin
| Treatment Group | # of Blastocysts | Total Cell Count | ICM Cell Count | TE Cell Count | ICM/TE Ratio |
|---|---|---|---|---|---|
| Control | 22 | 195.1 ± 20.3 | 36.1 ± 3.5 | 159.0 ± 18.6 | 0.275 ± 0.035 |
| Relaxin | 21 | 202.0 ± 18.9 | 35.8 ± 2.6 | 166.2 ± 18.9 | 0.273 ± 0.036 |
Values are Mean ± SEM.
DISCUSSION
We show here the first evidence that the relaxin pathway plays a role in primate pre-implantation embryo development. Our data demonstrate low-level expression of the RXFP1 mRNA throughout pre-implantation development, and RXFP1 protein expression selectively in the ICM at the blastocyst stage. These results establish the potential for a response to relaxin in the preimplantation embryo. We observed that exogenous relaxin elicited a highly favorable response in vitro, manifested as a significant acceleration of developmental rate to blastocyst, which is widely correlated with improved embryo quality (28, 30). These results thus establish relaxin as a key factor for enhancing in vitro embryo development, and further suggest that relaxin exerts a similarly positive effect in vivo.
The effect of relaxin on embryo development could have important application for human clinical IVF programs. There is evidence that embryos cultured in vitro after IVF take longer to reach the blastocyst stage of development than their in vivo counterparts. Embryos that take longer to develop also have a lower implantation rate (31), but this effect may also involve poor timing for the endometrium as well as compromised embryo quality for slower developing embryos (32). In most human clinics, embryos reach the blastocyst stage on day 5 or 6 after retrieval (31) whereas in vivo produced embryos flushed from the reproductive tract become blastocysts on about day 4.5 following ovulation (33). Evidence for the relative delay in implantation has also been noted in the timing of increasing serum hCG when comparing spontaneous and IVF pregnancies (34). A similar delay in embryo development has been noted for rhesus monkey embryos obtained from in vitro culture compared to those from in vivo production (35, 36). Although the 7 to 11 day time to blastocyst formation reported by Zhang et al. (35) is generally longer than time to blastocyst in this study and others from this laboratory (28), all in vitro produced embryos take longer to develop than the 7 days for “hatched” blastocysts reported for in vivo embryos (35). If in vitro embryo culture can be advanced with the addition of relaxin, embryos could potentially be ready for implantation at a time that is more appropriately synchronized to the endometrium.
The level of relaxin chosen for this study has been effective in this range in a number of end points, from cell systems (37) to a recent clinical study (38). The level in this experiment is higher than the 50 pg/ml range that is circulating in the blood at the comparable time during the menstrual cycle or during an equivalent time in very early pregnancy (4, 5). However, the levels of relaxin in the reproductive tract may be much higher than that measured in peripheral blood at this time, which could be due to one or more mechanisms. Firstly, luteal secretion of relaxin at this time (5) might be transferred from the ovarian vein to the oviductal artery through countercurrent mechanisms. The vascular anatomy in the female reproductive tract of many species, including human and nonhuman primates, allows for a system of counter-current exchange where the ovary could products to the ovary, Fallopian tube and part of the uterus (39, 40 for reviews). Molecules as large as relaxin have been shown to be transferred from the ovarian vein to the ovarian artery (41). Secondly, the human fallopian tubal epithelium has been reported to be a source of relaxin as shown by expression of both message and protein (9). Additionally, peritoneal fluid has been found to contain relaxin immunoactivity during the mid-luteal phase which ranges up to 775 pg/ml (42).
The secretion of relaxin during the mid to late luteal phase of the menstrual cycle has been shown in humans (3, 41) and macaques (5). Because the profile of relaxin secretion during this time is different from that seen in rodents and domestic animals, the peri-implantational role of relaxin may be different from those species. Primate models that exhibit relaxin production during the menstrual cycle and early pregnancy can be used for future studies on the action of relaxin on preimplantation embryos and may have important application to human fertility and early embryonic development. Relaxin is a second factor recently shown to enhance in vitro primate embryo development (28). These breakthroughs may greatly augment what can be accomplished in human clinical infertility programs as well as in culturing embryos of other species, thus promoting agricultural advancement and endangered species
Acknowledgments
This research was supported by NIH grants RR13439 (CAV), RR00169 (CNPRC), and RR15253 (KEL)
We would like to express our gratitude to Bela Patel, Young Lee and Dana Hill for assistance.
Footnotes
Disclosure summary:
DRS is an employee of Corthera, Inc., a wholly owned subsidiary of Novartis, which is in commercial development of relaxin. All other authors have nothing to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hisaw FL. Experimental relaxation of the pubic ligament of the guinea pig. Proc Soc Exp Biol Med. 1926;23:661–3. [Google Scholar]
- 2.Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocr Rev. 2004 Apr;25(2):205–34. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- 3.Stewart DR, Celniker AC, Taylor CA, Jr, Cragun JR, Overstreet JW, Lasley BL. Relaxin in the peri-implantation period. J Clin Endocrinol Metab. 1990;70(6):1771–3. doi: 10.1210/jcem-70-6-1771. [DOI] [PubMed] [Google Scholar]
- 4.Stewart DR, Overstreet JW, Celniker AC, Hess DL, Cragun JR, Boyers SP, et al. The relationship between hCG and relaxin secretion in normal pregnancies vs peri-implantation spontaneous abortions. Clin Endocrinol (Oxf) 1993;38(4):379–85. doi: 10.1111/j.1365-2265.1993.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 5.Stewart DR, Stouffer R, Overstreet JW, Hendrickx A, Lasley BL. Measurement of periimplantational relaxin concentrations in the macaque using a homologous assay. Endocrinology. 1993;132(1):6–12. doi: 10.1210/endo.132.1.8419146. [DOI] [PubMed] [Google Scholar]
- 6.Stewart DR, VandeVoort CA. Simulation of human luteal function with granulosa lutein cell culture. J Clin Endocrinol Metab. 1997;82:3078–83. doi: 10.1210/jcem.82.9.4240. [DOI] [PubMed] [Google Scholar]
- 7.Hansell DJ, Bryant-Greenwood GD, Greenwood FC. Expression of the human relaxin H1 gene in the decidua, trophoblast, and prostate. J Clin Endocrinol Metab. 1991;72(4):899–904. doi: 10.1210/jcem-72-4-899. [DOI] [PubMed] [Google Scholar]
- 8.Yki-Jarvinen H, Wahlstrom T, Seppala M. Immunohistochemical demonstration of relaxin in the genital tract of pregnant and nonpregnant women. J Clin Endocrinol Metab. 1983;57(3):451–4. doi: 10.1210/jcem-57-3-451. [DOI] [PubMed] [Google Scholar]
- 9.Tang XM, Chegini N. Human fallopian tube as an extraovarian source of relaxin: messenger ribonucleic acid expression and cellular localization of immunoreactive protein and 125I-relaxin binding sites. Biol Reprod. 1995;52(6):1343–9. doi: 10.1095/biolreprod52.6.1343. [DOI] [PubMed] [Google Scholar]
- 10.Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, et al. Activation of orphan receptors by the hormone relaxin. Science. 2002;295(5555):671–4. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- 11.Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol Rev. 2006;58(1):7–31. doi: 10.1124/pr.58.1.9. [DOI] [PubMed] [Google Scholar]
- 12.Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. Receptors for relaxin family peptides. Ann N Y Acad Sci. 2005;1041:61–76. doi: 10.1196/annals.1282.010. [DOI] [PubMed] [Google Scholar]
- 13.Bani G, Maurizi M, Bigazzi M, Sacchi TB. Effects of relaxin on the endometrial stroma. Studies in mice. Biol Reprod. 1995;53:253–62. doi: 10.1095/biolreprod53.2.253. [DOI] [PubMed] [Google Scholar]
- 14.Einspanier A, Lieder K, Husen B, Ebert K, Lier S, Einspanier R, et al. Relaxin supports implantation and early pregnancy in the marmoset monkey. Ann N Y Acad Sci. 2009;1160:140–6. doi: 10.1111/j.1749-6632.2009.03947.x. [DOI] [PubMed] [Google Scholar]
- 15.Palejwala S, Tseng L, Wojtczuk A, Weiss G, Goldsmith LT. Relaxin gene and protein expression and its regulation of procollagenase and vascular endothelial growth factor in human endometrial cells. Biol Reprod. 2002;66:1743–1748. doi: 10.1095/biolreprod66.6.1743. [DOI] [PubMed] [Google Scholar]
- 16.Tang M, Mazella J, Zhu HH, Tseng L. Ligand activated relaxin receptor increases the transcription of IGFBP-1 and prolactin in human decidual and endometrial stromal cells. Mol Hum Reprod. 2005;11(4):237–43. doi: 10.1093/molehr/gah149. [DOI] [PubMed] [Google Scholar]
- 17.Maseelall PB, Seungdamrong A, Weiss G, Wojtczuk AS, Donnelly R, Stouffer RL, et al. Expression of LGR7 in the primate corpus luteum implicates the corpus luteum as a relaxin target organ. Ann N Y Acad Sci. 2009;1160:147–51. doi: 10.1111/j.1749-6632.2009.03946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyholt de Prada JK, VandeVoort CA. Growth hormone and in vitro maturation of rhesus macaque oocytes and subsequent embryo development. J Assist Reprod Genet. 2008;25:145–58. doi: 10.1007/s10815-008-9208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VandeVoort CA, Tarantal AF. The macaque model for in vitro fertilization: Techniques and sonographic applications. J Med Primatol. 1991;20:110–6. [PubMed] [Google Scholar]
- 20.VandeVoort CA, Leibo SP, Tarantal AF. Improved collection and developmental competence of immature macaque oocytes. Theriogenology. 2003;59:699–707. doi: 10.1016/s0093-691x(02)01129-9. [DOI] [PubMed] [Google Scholar]
- 21.Sarason RL, VandeVoort CA, Mader DR, Overstreet JW. The use of nonmetal electrodes in electroejaculation of restrained but unanesthetized macaques. J Med Primatol. 1991;20:122–5. [PubMed] [Google Scholar]
- 22.Boatman DE. In vitro growth of non-human primate pre- and peri- implantation embryos. In: Bavister BD, editor. The Mammalian Preimplantation Embryo: regulation of growth and differentiation in vitro. New York: Plenum Press; 1987. pp. 273–308. [Google Scholar]
- 23.McKiernan SH, Bavister BD. Culture of one-cell hamster embryos with water-soluble vitamins: pantothenate stimulates blastocyst production. Hum Reprod. 2000;15:157–64. doi: 10.1093/humrep/15.1.157. [DOI] [PubMed] [Google Scholar]
- 24.Parry LJ, Stewart DR, Kumar P, Ji J, Douglas GC. Relaxin Receptor Expression in the Rhesus Monkey Trophoblast in Early Implantation. Reproductive Sciences. 2010;17(Suppl S):3, 123A. [Google Scholar]
- 25.Kern A, Bryant-Greenwood GD. Characterization of relaxin receptor (RXFP1) desensitization and internalization in primary human decidual cells and RXFP1-transfected HEK293 cells. Endocrinology. 2009;150(5):2419–28. doi: 10.1210/en.2008-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allworth AE, Albertini DF. Meiotic maturation in cultured bovine oocytes is accompanied by remodeling of the cumulus cell cytoskeleton. Dev Biol. 1993;158(1):101–12. doi: 10.1006/dbio.1993.1171. [DOI] [PubMed] [Google Scholar]
- 27.Halls ML, Bathgate RA, Summers RJ. Relaxin family peptide receptors RXFP1 and RXFP2 modulate cAMP signaling by distinct mechanisms. Mol Pharmacol. 2006;70(1):214–26. doi: 10.1124/mol.105.021691. [DOI] [PubMed] [Google Scholar]
- 28.VandeVoort CA, Mtango NR, Lee YS, Smith GW, Latham KE. Differential Effects of Follistatin on Nonhuman Primate Oocyte Maturation and Pre-Implantation Embryo Development In Vitro. Biol Reprod. 2009;81(6):1139–46. doi: 10.1095/biolreprod.109.077198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards RG, Fishel SB, Cohen J, Fehilly CB, Purdy JM, Slater JM, et al. Factors influencing the success of in vitro fertilization for alleviating human infertility. J In Vitro Fert Embryo Transf. 1984;1:3–23. doi: 10.1007/BF01129615. [DOI] [PubMed] [Google Scholar]
- 30.Van Montfoort AP, Dumoulin JC, Kester AD, Evers JL. Early cleavage is a valuable addition to existing embryo selection parameters: a study using single embryo transfers. Hum Reprod. 2004;19:2103–8. doi: 10.1093/humrep/deh385. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro BS, Richter KS, Harris DC, Daneshmand ST. A comparison of day 5 and day 6 blastocyst transfers. Fertil Steril. 2001;75(6):1126–30. doi: 10.1016/s0015-0282(01)01771-x. [DOI] [PubMed] [Google Scholar]
- 32.Richter KS, Shipley SK, McVearry I, Tucker MJ, Widra EA. Cryopreserved embryo transfers suggest that endometrial receptivity may contribute to reduced success rates of later developing embryos. Fertil Steril. 2006;86(4):862–6. doi: 10.1016/j.fertnstert.2006.02.114. [DOI] [PubMed] [Google Scholar]
- 33.Buster JE, Bustillo M, Rodi IA, Cohen SW, Hamilton M, Simon JA. Biologic and morphologic development of donated human ova recovered by nonsurgical uterine lavage. Am J Obstet Gynecol. 1985;153:211–7. doi: 10.1016/0002-9378(85)90116-4. [DOI] [PubMed] [Google Scholar]
- 34.Tulchinsky D, Tulchinsky A, Paoletti-Falcone V, Nash H, Pazdziorko S, Brown K. Delayed embryo implantation following in vitro fertilization and embryo transfer (IVFET) J Assist Reprod Genet. 1996;13(7):536–9. doi: 10.1007/BF02066604. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Weston AM, Denniston RS, Goodeaux LL, Godke RA, Wolf DP. Developmental potential of rhesus monkey embryos produced by in vitro fertilization. Biol Reprod. 1994;51(3):433–40. doi: 10.1095/biolreprod51.3.433. [DOI] [PubMed] [Google Scholar]
- 36.Enders AC, Schlafke S. Differentiation of the Blastocyst of the rhesus monkey. Am J Anat. 1981;162(1):1–21. doi: 10.1002/aja.1001620102. [DOI] [PubMed] [Google Scholar]
- 37.Unemori EN, Erikson ME, Rocco SE, Sutherland KM, Parsell DA, Mak J, et al. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod. 1999;14(3):800–6. doi: 10.1093/humrep/14.3.800. [DOI] [PubMed] [Google Scholar]
- 38.Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet. 2009;373(9673):1429–39. doi: 10.1016/S0140-6736(09)60622-X. [DOI] [PubMed] [Google Scholar]
- 39.Einer-Jensen N, Hunter R. Physiological and pharmacological aspects of local transfer of substances in the ovarian adnexa in women. Hum Reprod Update. 2000;6(2):132–8. doi: 10.1093/humupd/6.2.132. [DOI] [PubMed] [Google Scholar]
- 40.Einer-Jensen N, Hunter R. Counter-current transfer in reproductive biology. Reproduction. 2005;129(1):9–18. doi: 10.1530/rep.1.00278. [DOI] [PubMed] [Google Scholar]
- 41.Schramm W, Einer-Jensen N, Schramm G. Direct venous-arterial transfer of 125I-radiolabelled relaxin and tyrosine in the ovarian pedicle in sheep. J Reprod Fertil. 1986;77(2):513–21. doi: 10.1530/jrf.0.0770513. [DOI] [PubMed] [Google Scholar]
- 42.Loumaye E, Depreester S, Donnez J, Thomas K. Immunoreactive relaxin surge in the peritoneal fluid of women during the midluteal phase. Fertil Steril. 1984;42(6):856–60. doi: 10.1016/s0015-0282(16)48256-7. [DOI] [PubMed] [Google Scholar]
- 43.Stewart DR, Nakajima ST, Overstreet J, Boyers S, Lasley B. Relaxin as a Biomarker for Human Pregnancy Detection. In: MacLennan A, Tregear G, Bryant-Greenwood G, editors. Progress in Relaxin Research. Singapore: World Scientific Publishing Co; 1995. pp. 214–24. [Google Scholar]


