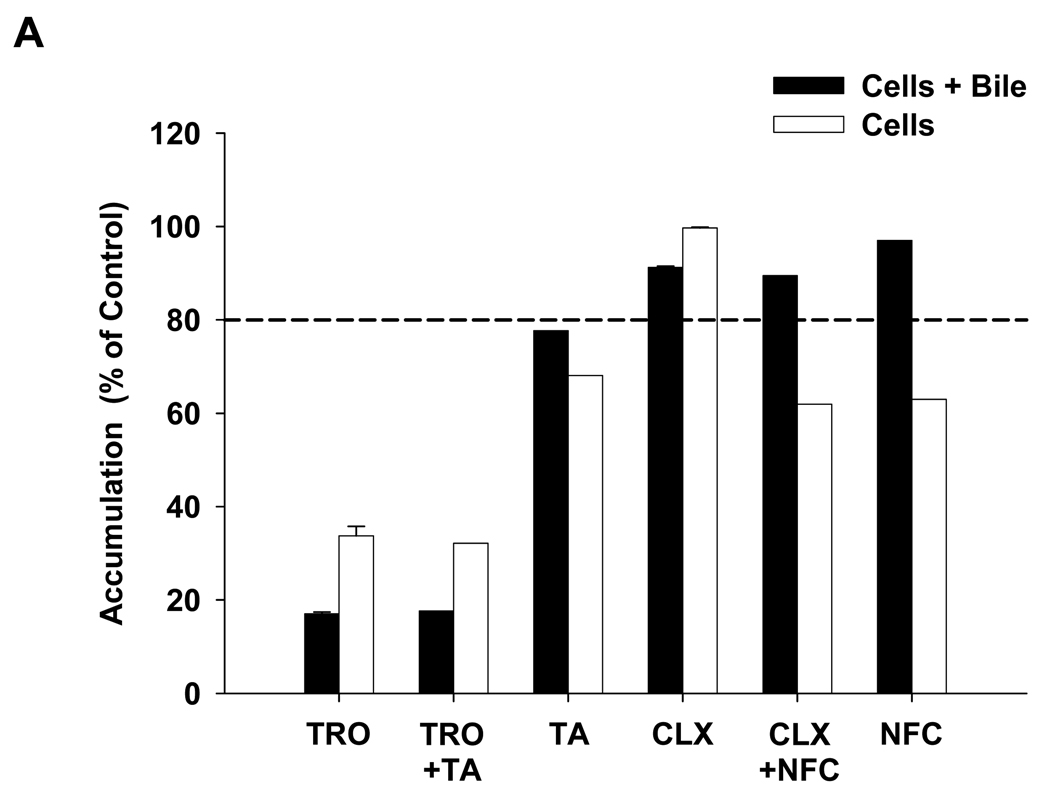
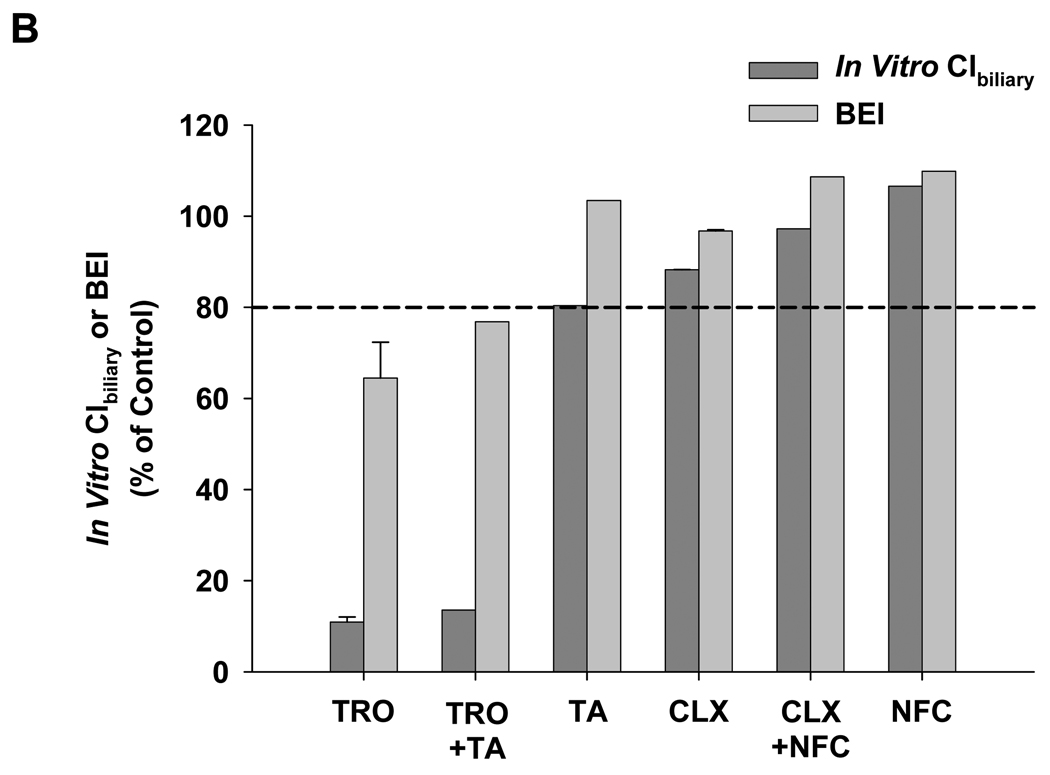
Figure 10. Effect of hepatotoxic compounds on the hepatobiliary disposition of taurocholate in human SCH.
Human SCH were incubated with [3H]-taurocholate and the hepatotoxic compound(s) of interest for 10 min following a 10-min incubation in standard or Ca2+-free buffer containing the same hepatotoxic compound(s). (A) Taurocholate accumulation. Solid bars represent accumulation in hepatocytes and bile canaliculi (cells+bile). Open bars represent accumulation in hepatocytes (cells). (B) BEI and in vitro Clbiliary were calculated as described in Materials and Methods. Data are presented as the mean for n=1 liver in duplicate, except for troglitazone and cloxacillin (mean ± ½ of the range for n=2 livers in duplicate). TRO, troglitazone; TA, tienilic acid (3.00 µM); CLX, cloxacillin; NFC, nafcillin. Data for treatment with troglitazone, cloxacillin, and nafcillin alone also were presented in Table 3 and serve as a reference in this figure.