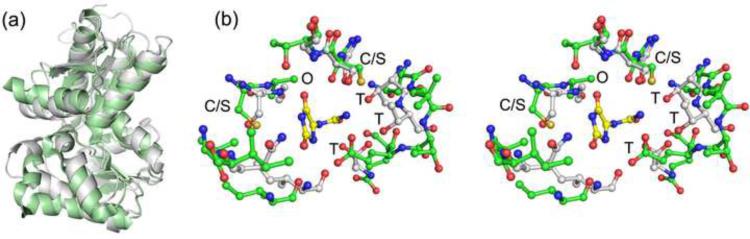
Fig. 4.
Superposition of KpHpxA and the hydantoin racemase from P. horikoshii (2EQ5). (a) Secondary structural superposition of the protomers from KpHpxA (white) and 2EQ5 (green). (b) Stereodiagram of the superposition of the active sites of KpHpxA (white carbon atoms, blue nitrogen atoms, and red oxygen atoms) and 2EQ5 (green carbon atoms) with allantoin shown (yellow carbon atoms). The two active site cysteines (serines in KpHpxA-allantoin structure) are labeled with C/S, the putative oxyanion hole is designated with an O, and each of the threonine residues that interact with the 'tail' region of allantoin in KpHpxA is labeled with T.