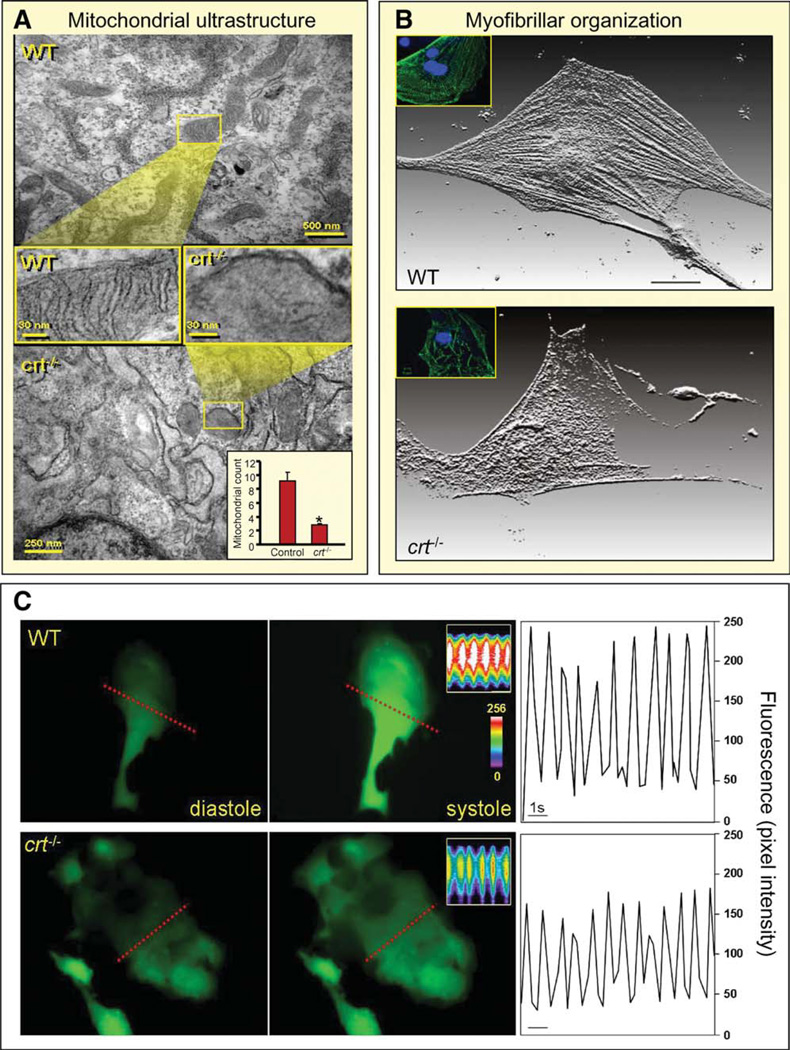
Figure 6.
Embryonic transcriptome shift precipitated by calreticulin deletion translates into phenotype derangement. (A): Comparison of mitochondria, visualized by electron microscopy, in WT (upper) and calreticulin-deficient embryonic stem cells (lower). Cross-sectional magnification revealed absence of cristae in the latter. Inset, lower right: Enumeration of mitochondrial number in WT versus crt−/−. Mitochondrial count indicated on y-axis. *, p < .05, n = 5. (B): Individual stem cell-derived cardiomyocytes stained for α-actinin, displayed here as a three-dimensional reconstruction of fluorescence. Proper sarcomere formation in WT stem cell-derived cardiomyocytes is illustrated (upper), and disarrayed myofibrillogenesis or no sarcomere generation is present in cardiomyocytes from crt−/− stem cells. Inset: Stem cell-derived cardiomyocytes stained for MLC2V (green) and DAPI-counterstained nuclei (blue). (C): Calcium transient measurements. Left and middle panels: Ca2+ levels in diastole and systole in WT and crt−/− stem cell-derived cardiomyocytes loaded with the calcium indicator, Fluo-3. Inset: Line scan (indicated by dashed line) records calcium transients in contractile cells. Color scale indicates high (white) and low (blue/purple) calcium concentrations. Right: Analysis of calcium transients as a function of time demonstrate attenuated peak intensity in crt−/− knockouts (bottom) compared with WT counterparts (top). Bar = 1 second. Abbreviation: WT, wild type.