Abstract
Synaptic GTPase-activating protein (SynGAP) is a neuronal-specific Ras/Rap-GAP that increases the hydrolysis rate of GTP to GDP, converting Ras/Rap from the active into the inactive form. The Ras protein family modulates a wide range of cellular pathways including those involved in sensitization of sensory neurons. Since GAPs regulate Ras activity, SynGAP might be an important regulator of peripheral sensitization and pain. Therefore, we evaluated excitability, stimulus-evoked release of the neuropeptide calcitonin gene-related peptide (CGRP), and nociception from wild-type (WT) mice and those with a heterozygous mutation of the SynGAP gene (SynGAP+/−). Our results demonstrate that SynGAP is expressed in primary afferent sensory neurons and that the capsaicin-stimulated CGRP release from spinal cord slices was two-fold higher from SynGAP+/− mice than that observed from WT mouse tissue, consistent with an increase in expression of the capsaicin receptor, transient receptor potential cation channel subfamily V member 1 (TRPV1), in SynGAP+/− dorsal root ganglia. However, there was no difference between the two genotypes in potassium-stimulated release of CGRP, the number of action potentials generated by a ramp of depolarizing current, or mechanical hypernociception elicited by intraplantar injection of capsaicin. In contrast, capsaicin-induced thermal hypernociception occurred at lower doses of capsaicin and had a longer duration in SynGAP+/− mice than WT mice. These results provide the first evidence that SynGAP is an important regulator of neuropeptide release from primary sensory neurons and can modulate capsaicin-induced hypernociception, demonstrating the importance of GAP regulation in signaling pathways that play a role in peripheral sensitization.
Keywords: calcitonin gene-related peptide, capsaicin, dorsal root ganglia, thermal hypernociception
the ras family of small G-proteins mediates a number of downstream signaling pathways that regulate cellular functions, including alterations in gene expression and post-translational modification of proteins (Donovan et al. 2002; Karnoub and Weinberg 2008). In sensory neurons, these alterations can result in an enhanced excitability that produces increased sensitivity to noxious stimuli (Ji and Woolf 2001; Nicol and Vasko 2007). Indeed, a number of putative inflammatory mediators, including cytokines and NGF, can activate the Ras/MEK/ERK signaling pathway in sensory neurons (Hensellek et al. 2007; Nicol and Vasko 2007; Zhuang et al. 2004), and the consequences of long-term activation of this pathway are the subject of much study. Several investigations have shown that phosphorylation and activation of ERK are involved in peripheral sensitization (Dai et al. 2002; Ji et al. 1999; Obata et al. 2003; Zhuang et al. 2004). Furthermore, there is evidence that activation of the Ras/Raf/MEK/ERK pathway mediates neurotrophin-induced increases in expression of transient receptor potential cation channel subfamily V member 1 (TRPV1) (Bron et al. 2003).
Although Ras is activated by various tyrosine kinase receptors, it is negatively regulated by GTPase-activating proteins (GAPs), which increase the hydrolysis rate of GTP to GDP, therefore converting Ras from active Ras-GTP to inactive Ras-GDP (Scheffzek et al. 1998). Thus GAPs can be critical proteins in regulating the peripheral sensitization and nociception mediated by Ras signaling cascades. Indeed, we previously demonstrated that primary sensory neurons from mice with reduced expression of neurofibromin, another Ras-GAP, exhibit increased excitability and sensitivity to chemical stimulation (Hingtgen et al. 2006; Wang et al. 2005).
In addition to neurofibromin, a neuronal Ras/Rap-GAP, called synaptic GAP (SynGAP), was first described at excitatory synapses of the central nervous system (CNS) (Chen et al. 1998; Kim et al. 1998). This GAP plays an important role in models of synaptic plasticity (Kim et al. 2003; Komiyama et al. 2002; Rumbaugh et al. 2006), apoptosis (Knuesel et al. 2005), and schizophrenia (Guo et al. 2009). SynGAP is associated with proteins in the postsynaptic density, including postsynaptic density-95 kDa and the N-methyl-d-aspartate (NMDA) receptor (Chen et al. 1998; Kim et al. 1998), and plays a crucial role in early development, since SynGAP knockout mice die within 1 wk of birth (Kim et al. 1998). Mice with a heterozygous mutation of SynGAP (SynGAP+/−) have a significant reduction in long-term potentiation and an increased expression of phosphorylated (p)-ERKs (Kim at al. 2003; Komiyama et al. 2002). The question remains whether SynGAP plays a regulatory role in the peripheral nervous system (PNS) and specifically, if this GAP is involved in regulating the function of peripheral sensory neurons important in nociception. To address this question, we examined the consequences of reduced SynGAP expression on three important functional outputs of sensory neurons: 1) stimulus-evoked release of neuropeptides, 2) membrane excitability, and 3) capsaicin-induced hypernociception. Our results demonstrate that reduced SynGAP expression results in an increase in capsaicin sensitivity—the first evidence that SynGAP is an important regulator for peripheral neuronal sensitization.
METHODS
Animals.
SynGAP+/− mice, on a background of C57BL/6J (25–30 g), were generously donated by Dr. Richard Huganir (Johns Hopkins University, Baltimore, MD), bred in the Indiana University Laboratory Animal Research Center (Indianapolis, IN), and used in accordance with National Institutes of Health Guide for Care and Use of Laboratory Animals. All procedures were reviewed and approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. Food and water were available ad libitum. With the use of the protocol of Kim et al. (2003), all animals were genotyped prior to use in experiments.
Materials.
Capsaicin, 1-methyl-2-pyrrolidone (MPL), and phosphatase inhibitors cocktails 1 and 2 were from Sigma (St. Louis, MO), and protease inhibitor cocktail set I was from Calbiochem (San Diego, CA).
For Western blot assays, the following antibodies were used: rabbit anti-SynGAP antibody from Abcam (Cambridge, MA; catalog #ab277235), goat anti-TRPV1 from Santa Cruz Biotechnology (Santa Cruz, CA; catalog #sc12498), mouse anti-GAPDH from Chemicon International (Billerica, MA; catalog #MAB374), mouse anti-actin from Thermo Scientific (Fremont, CA; catalog #MS1295-P), rabbit anti-p-ERK1/2 and anti-ERK1/2 from Cell Signaling Technology (Danvers, MA; catalog #4376 and #4695), goat anti-rabbit horseradish peroxidase (HRP)-conjugated IgG secondary antibody from Bio-Rad (Hercules, CA; catalog #1721019), and donkey anti-goat and goat anti-mouse HRP-conjugated IgG secondary antibody from Jackson ImmunoResearch Labs (West Grove, PA; catalog #705-035-003 and #11-035-003).
For the immunohistochemistry studies, goat anti-peripherin was from Santa Cruz Biotechnology (catalog #sc-7604), and donkey anti-goat secondary antibody conjugated to AlexaFluor488 and goat anti-rabbit antibody conjugated to AlexaFlour568 were from Molecular Probes (Carlsbad, CA; catalog #A11055 and #A11011).
Isolation of sensory neurons from dorsal root ganglia.
Primary cultures of dorsal root ganglia (DRG) cells were established as previously described (Hingtgen et al. 2006; Wang et al. 2005). Briefly, adult mice were killed by CO2 asphyxiation, and all of the DRG were collected and incubated for 8–12 min in Puck's saline containing 10 U/ml papain II at 37°C. After centrifugation, the tissue was resuspended and incubated at 37°C for 8–12 min in Puck's saline containing collagenase (1 mg/ml; type 1A) and dispase II (2.5 mg/ml). After digestion, the DRG were mechanically dissociated in F-12 media, and the isolated cells were plated onto plastic coverslips, which were previously coated with poly-d-lysine and laminin. The cells were maintained in F-12 media in the absence of NGF at 37°C and 3% CO2 and used within 12–24 h for electrophysiological recordings.
Immunocytochemistry.
Cells for immunhistochemistry were plated on Lab-Tek microscope chamber slides coated with poly-d-lysine and laminin and maintained in F-12 media supplemented with 2 mM glutamine, 50 μg/ml penicillin, and streptomycin, 10% heat-inactivated horse serum, mitotic inhibitors (50 μM 5-fluoro-2-deoxyuridine and 150 μM uridine), and 30 ng/ml NGF. Growth medium was changed every 2–3 days, and cells were used 5–7 days after plating. The chamber slides were washed twice with PBS at room temperature. The cells were fixed by incubating in 4% paraformaldehyde in PBS for 20 min at room temperature, blocked with 4% normal goat serum and 0.3% Triton X-100 in PBS solution for 1 h at room temperature, and incubated overnight at 4°C with peripherin (1:250) and SynGAP (1:500) antibody. The slides then were incubated for 1 h with a 1:500 dilution of donkey anti-goat secondary antibody conjugated to AlexaFluor488 or goat anti-rabbit conjugated to AlexaFluor568. Fluorescent images were acquired at the Indiana Center for Biological Microscopy using a Bio-Rad MRC-1024MP laser-scanning confocal/multiphoton scanner attached to a Nikon TE-200 inverted microscope with a Nikon ×60/1.2-NA water-immersion objective. Fluorescence excitation (488, 568, and 647 nm) was provided by a krypton-argon laser, and images were captured in black and white to maximize resolution and pseudocolored to red (SynGAP) and green (peripherin) using Adobe Photoshop 7.0 (Adobe, Mountain View, CA).
Immunoblotting.
For Western blots, DRG from adult wild-type (WT) and SynGAP+/− animals were collected and homogenized with ice-cold, modified radioimmunoprecipitation assay buffer (RIPA; 50 mM Trizma base, 150 mM NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mM EDTA) with protease inhibitor cocktail set I (1:100) and phosphatase inhibitors cocktails 1 and 2 (1:100). Total protein was determined in each sample by Bradford assay. Samples were loaded onto a 10% or 4–12% NuPAGE bis-tris gel, and electrophoresis was performed at 200 V. Proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane at 30 V for 1 h. PVDF membranes were blocked in Tris-buffered saline (20 mM Tris, 500 mM NaCl, pH 7.5) containing 0.1% Tween 20 (TBST) and 5% nonfat dry milk for 1 h at room temperature and then incubated with primary antibody for SynGAP (1:500), p-ERK1/2 (1:500), ERK1/2 (1:1,000), TRPV1 (1:500), GADPH (1:3,000), or actin (1:5,000) in TBST with 5% nonfat dry milk overnight at 4°C, washed, and incubated with corresponding secondary antibody for 1 h at room temperature. After repeated washing, the proteins were visualized by the Western Lightning Plus-ECL enhanced chemiluminescent HRP substrate and autoradiographic film. Signals present on film were scanned into the Adobe Photoshop 7.0 program using a Hewlett-Packard Scanjet 5470c scanner (Palo Alto, CA), and densitometric analysis was performed using Quantity One software from Bio-Rad. The SynGAP signal was normalized to the signal from the loading control, GAPDH. The TRPV1 signal was normalized to the signal from the loading control, actin, and the p-ERK signal was normalized to the total ERK signal.
Electrophysiological recording.
Electrophysiological recordings were made using the whole-cell patch-clamp technique as previously described (Wang et al. 2005). A coverslip was placed in a recording chamber where the neurons were bathed in normal Ringer solution containing (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose; pH was adjusted to 7.4 with NaOH. Recording pipettes typically had resistances of 2–4 MΩ when filled with the following solution (in mM): 140 KCl, 5 MgCl2, 4 ATP, 0.3 GTP, 2.5 CaCl2, 5 EGTA (calculated free Ca2+ concentration of 100 nM), and 10 HEPES; pH was adjusted to 7.3 with potassium hydroxide. Whole-cell voltages or currents were recorded with an Axopatch 200B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA). The data were acquired and analyzed using pCLAMP 9.2 or Clampfit 9.2 (Molecular Devices). The whole-cell recording configuration was established in normal Ringer solution. To measure excitability in the current clamp experiments, DRG neurons were held at their resting potentials, and a depolarizing ramp of current (1 s in duration) was applied. The amplitude of the ramp current was 200, 500, and 1,000 pA. At the end of the recordings, neurons were exposed to 0.5–1 μM capsaicin. All patch-clamp recordings were performed on small diameter (<25 μm) sensory neurons. All experiments were performed at room temperature (∼23°C).
Release of immunoreactive calcitonin gene-related peptide from mouse spinal cord tissue.
The release of immunoreactive calcitonin gene-related peptide (iCGRP) from spinal cord tissue was performed using a modification of our technique as previously described (Chen et al. 1996; Hingtgen et al. 2006). Briefly, after adult mice were killed using CO2 asphyxiation and decapitation, the spinal cord from each animal was removed, weighed, and chopped parasagitally and transversely into 300-μm pieces using a McIlwain tissue chopper. The sections from each spinal cord were placed into individual chambers and perfused at a flow rate of 0.5 ml/min with a Hepes buffer, consisting of Hepes 25 mM, NaCl 135 mM, KCl 3.5 mM, MgSO4 1 mM, CaCl2 2.5 mM, dextrose 3.3 mM, BSA 1%, ascorbic acid 200 μM, Phe-Ala 100 μM, and bacitracin 20 μM, aerated with 95% O2–5% CO2, pH 7.4–7.5, and maintained at 36–37°C. After 20 min, the flow was switched to 0.1 ml/min for collection of perfusate samples, and 1-ml samples were collected into test tubes containing 75 μl of 1 M MES buffer (pH 6.7–6.9) every 10 min. Basal release was established by perfusing the tissue with Hepes buffer for 30 min. To evoke the release of iCGRP, the tissue was perfused for an additional 20 min with Hepes buffer containing 500 nM capsaicin or 50 mM KCl, substituted for equimolar NaCl. After stimulation, the tissue was perfused with Hepes buffer alone for another 40 min to assure return to basal levels. After the release protocol was complete, the spinal cord tissue was collected and homogenized in 4 ml 0.1 N HCl. The homogenate was centrifuged at 3,000 g for 20 min at 4°C, and the supernatant was serially diluted with Hepes buffer and assayed for iCGRP by radioimmunoassay, as previously described (Hingtgen and Vasko 1994; Vasko et al. 1994). The total peptide content was calculated as the amount of iCGRP released during the entire perfusion, plus the amount remaining in the tissues after the perfusion was complete. The amount of iCGRP released/min in each perfusion fraction divided by the total peptide content for that sample is presented as percent of content/min of perfusion. Evoked release is calculated as the cumulative release over the 20 min of tissue exposure to capsaicin or high extracellular potassium minus the cumulative release for the 20-min period prior to stimulation, expressed as percent of the total iCGRP content.
Nociceptive tests.
Mechanical hypernociception was evaluated using Von Frey filaments (Stoelting, Kiel, WI). Mice were placed in an elevated cage with a wire-mesh bottom, which allowed easy access to the plantar surface of the hindpaw. To allow behavioral accommodation, prior to the initial trial, animals were placed in individual transparent cubicles with an opaque divider between each cubicle. When first placed in the cubicles, the animals presented a very common exploratory behavior, during which, it was not possible to apply either Von Frey filaments or radiant heat stimuli. After 30 min, the exploratory behavior decreases significantly, and the animals' behavioral state varies among grooming, alertness, or resting. The tests were applied only when the animal was not grooming. The paw was touched with one of a series of filaments with logarithmically incremental stiffness (0.407–8 g, lower and upper limit of the test, respectively). Each filament was applied from underneath through the mesh, vertically to the plantar surface, with sufficient force to cause a slight deflection of the filament. The location of testing was always at the center of the paw. A single trial consisted of three applications of a particular filament, applied once every 3–4 s. A response was defined as withdrawal of the stimulated paw. In the absence of a response to a particular filament, the next-stronger filament was used; in the case of a response, the next-weaker filament was presented. The up-down method was used to record the threshold (Chaplan et al. 1994).
Paw-withdrawal latency to radiant heat was assessed using a high-intensity bulb heat-source device (Ugo Basile, Comerio, Italy) (Hargreaves et al. 1988). Before each test, the animals remained in the test cage for ∼30 min to allow behavioral accommodation. The heat source was placed underneath the midplantar surface of the hind paw. The intensity of the heat source was adjusted to yield baseline latencies ranging from 8 s to 10 s, and a cut-off of 20 s was used to avoid tissue damage. The bulb and the electronic timer were switched off automatically when the photocell detected paw withdrawal in response to heat. The withdrawal latency was taken to be the mean of three trials, with ∼45 s between each trial. After baseline testing, capsaicin or vehicle (10 μl) was injected into the plantar surface of the paw. Capsaicin was initially dissolved in 0.5% MPL and diluted to the appropriate concentration in saline. Thresholds were measured 15, 30, 45, 60, and 120 min after drug administration. All tests occurred between 9:00 AM and 4:00 PM. In all experiments, the investigator was blinded to the genotype of the subject mouse.
Statistical analysis.
Results are presented as the mean ± SEM, and statistical analysis was performed using Student's t-test or two-way repeated-measures ANOVA, followed by post-hoc testing, as indicated. The level for significance in all tests was P < 0.05.
RESULTS
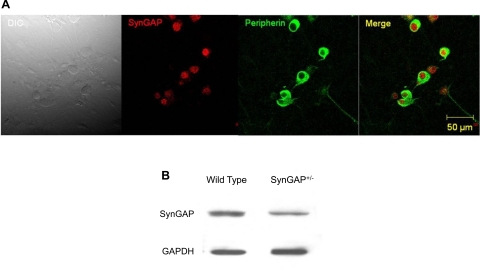
To determine if SynGAP is expressed in the PNS, confocal microscopy was used to ascertain whether SynGAP-like immunoreactivity was present in adult DRG sensory neurons grown in culture. As can be seen in Fig. 1A, SynGAP-like immunoreactivity was observed in neurons from WT mice and colocalized with the neuronal marker, peripherin. Furthermore, protein expression was confirmed using immunobloting of DRG extracts from WT and SynGAP+/− mice (Fig. 1B).
Fig. 1.
Expression of synaptic GTPase-activating protein (SynGAP) in peripheral sensory neurons. A: immunofluorescent staining of dissociated dorsal root ganglia (DRG) sensory neurons [from wild-type (WT) mice] showing that SynGAP (red) is present in neurons, also marked with the neuronal marker, peripherin (green). The horizontal bar represents 50 μm, and the magnification is 40×. DIC, differential interference contrast. B: immunoblots of DRG extracts showing SynGAP expression in WT and heterozygous mutation of the SynGAP gene (SynGAP+/−) mice.
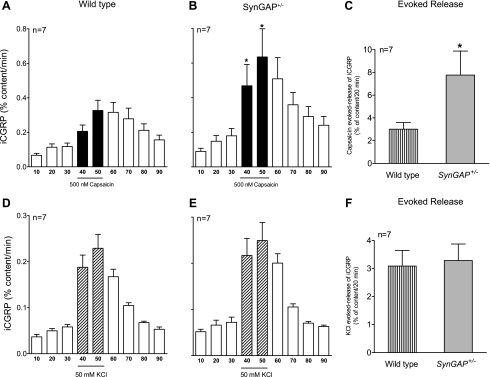
We previously demonstrated that sensory neurons with decreased expression of the Ras-GAP, neurofibromin, exhibit increased stimulus-evoked release of peptide transmitters (Hingtgen et al. 2006). To evaluate whether reduced SynGAP expression alters release of iCGRP, stimulus-evoked release of the neuropeptide was measured from spinal cord tissue preparations that contain the central terminals of the primary sensory neurons. There was no significant difference in resting release of iCGRP between WT and SynGAP+/− tissue, as indicated in Fig. 2, A and B. However, the evoked release of iCGRP (calculated by subtracting the resting release for 20 min prior to capsaicin exposure from the capsaicin-stimulated release) was significantly greater in the SynGAP+/− mice compared with WT littermates (3.01 ± 0.57% content/20 min for WT and 7.76 ± 2.1% content/20 min for SynGAP+/−; Fig. 2C). This enhancement was not the result of increased CGRP production, since the total content of iCGRP in the spinal cord tissue from the two genotypes was not different (136 ± 19 fmol/mg tissue for WT and 99.2 ± 23 fmol/mg tissue for SynGAP+/− mice).
Fig. 2.
Capsaicin- and potassium-stimulated release of immunoreactive calcitonin gene-related peptide (iCGRP) from spinal cord tissue of WT and SynGAP+/− mice. A, B, D, E: each column represents mean ± SEM of iCGRP release expressed as percent peptide content/min of perfusion from spinal cord tissues taken from WT mice or SynGAP+/− mice. The open columns represent release when tissue is perfused with HEPES buffer for successive 10-min intervals, whereas the black columns (A and B) or hatched columns (D and E) represent release when tissues are perfused with HEPES buffer containing 500 nM capsaicin or 50 mM potassium, respectively. C, F: the evoked release of iCGRP is calculated by subtracting the basal release for 20 min prior to stimulus exposure from the capsaicin- or potassium-stimulated release in WT or SynGAP+/− mice. *Significant difference (P < 0.05) between genotypes using Student's t-test.
Because capsaicin activates only a subpopulation of peptide-containing sensory neurons, we also examined if the response to the more general stimulus of high extracellular potassium was altered in mice with reduced SynGAP expression. As illustrated in Fig. 2, D and E, the reduction of expression of SynGAP did not alter the basal release of iCGRP. Moreover, there was no difference in the potassium-evoked release of iCGRP between SynGAP+/− and WT animals (3.08 ± 0.55% content/20 min for WT and 3.28 ± 0.58% content/20 min for SynGAP+/−; Fig. 2F). Similarly, there was no difference in the total content of iCGRP in the spinal cord tissue from the two genotypes (173.4 ± 19 fmol/mg tissue for WT and 167.1 ± 24 fmol/mg tissue for SynGAP+/−).
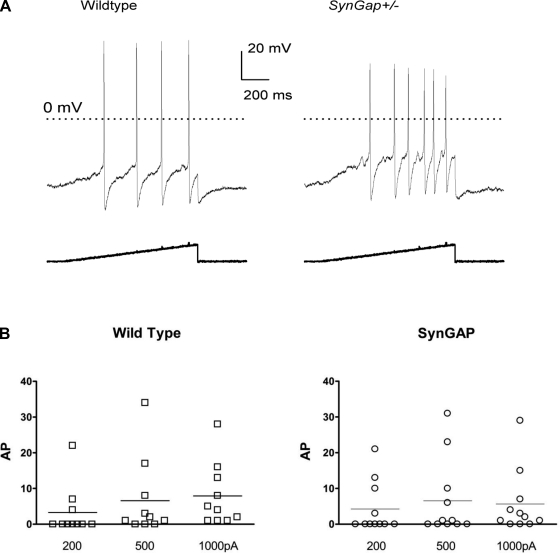
Action potential (AP) generation is one of the most fundamental processes in neuronal activity, and its modulation has a significant impact on neuronal plasticity and sensitivity. To determine whether reduced levels of SynGAP expression alter the excitability of small-diameter sensory neurons, the number of APs elicited by depolarizing ramps of current of different amplitudes (200, 500, and 1,000 pA) was evaluated. A representative pair of neurons exposed to a 200-pA ramp of current over 1 s is shown in Fig. 3A. Fig. 3B summarizes the results from 10 WT neurons (from nine mice) and 11 SynGAP+/− neurons (from nine mice), all of which were shown to be capsaicin sensitive at the end of the protocol. The 200-pA ramp elicited an average of 3.3 ± 2.2 APs and 3.0 ± 2.2 APs in the WT and SynGAP+/− group, respectively. The 500-pA ramp elicited an average of 6.6 ± 3.5 APs and 6.5 ± 3.2 APs in the WT and SynGAP+/− group, respectively. Finally, the 1,000-pA ramp elicited an average of 7.9 ± 2.8 APs and 5.6 ± 2.7 APs in the WT and SynGAP+/− group, respectively. There was no significant difference in the number of APs generated between the two genotypes for any of the ramp protocols tested.
Fig. 3.
SynGAP+/− sensory neurons do not exhibit increased neuronal firing compared with WT neurons. A: the number of action potentials (APs) of the 2 representative sensory neurons (1 from each genotype) in response to a 200-pA ramp of depolarizing current lasting 1 s. B: summarization of the APs induced by a 1-s ramp of depolarizing current with different amplitudes (200, 500, and 1,000 pA). The horizontal lines depict the mean number of APs in each group from 10 WT and 11 SynGAP+/− sensory neurons. There was no statistically significant difference between the number of APs generated in the 2 genotypes by any of the ramp protocols using Student's t-test (P > 0.05).
Besides the number of APs, the firing threshold and rheobase were measured as additional indices of neuronal excitability. The firing threshold is the membrane voltage at which the AP is generated, and the rheobase is the minimum amount of current required to evoke an AP. Neither the firing threshold (−14.7 ± 1.8 mV for WT vs. −15.8 ± 1.3 mV for SynGAP+/− neurons) nor the rheobase (175 ± 50 pA for WT vs. 181 ± 56 pA for SynGAP+/− neurons) was significantly different between the two genotypes. In addition, there was no significant difference in the average resting membrane potentials between the two genotypes (−52.5 ± 1.4 mV and −51.5 ± 1.1 mV for WT and SynGAP+/− neurons, respectively). These results demonstrate that reduced expression of SynGAP has no effect on the excitability of small-diameter sensory neurons.
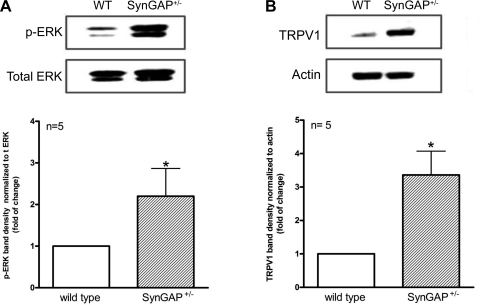
Because SynGAP facilitates conversion of active, GTP-bound Ras to the inactive, GDP-bound form, the increase in capsaicin-induced iCGRP release observed in sensory neurons from SynGAP+/− mice may result from increased levels of Ras-GTP activation and subsequent enhancement of downstream signaling pathways such as MEK/ERK. Thus we investigated whether the activity of the MEK/ERK pathway was altered in SynGAP+/− mice. As shown in Fig. 4A, the amount of immunoreactive p-ERK was approximately twofold higher in DRG extracts from SynGAP+/− mice than from DRG extracts of WT mice. This increase in p-ERK levels suggests an increase in MEK/ERK pathway activation, particularly as there was no difference in total ERK immunoreactivity between tissues from the two genotypes.
Fig. 4.
Decreased SynGAP expression results in enhanced phosphorylated (p)-ERK and transient receptor potential cation channel subfamily V member 1 (TRPV1) expression. A: representative blot from DRG protein extracts from WT or SynGAP+/− mice probed for p-ERK and total ERK (t ERK). Each column represents the density of the immunoreactive p-ERK band normalized to the total ERK band (mean ± SEM for 5 independent experiments). B: a representative blot from DRG protein extracts from WT or SynGAP+/− mice probed for TRPV1 and actin. Each column represents the density of the immunoreactive TRPV1 band normalized to the actin band. *Statistically significant difference between genotypes using Student's t-test.
Surprisingly, capsaicin, but not extracellular potassium, evoked the augmented release of iCGRP from SynGAP+/− neurons compared with WT. This raises the question as to whether reduced expression of SynGAP altered the expression of the capsaicin receptor, TRPV1. Indeed, when the expression of TRPV1 in DRG extracts from each genotype was compared, SynGAP+/− mice showed an approximate threefold increase in TRPV1 immunoreactivity compared with WT mouse DRG extracts (Fig. 4B).
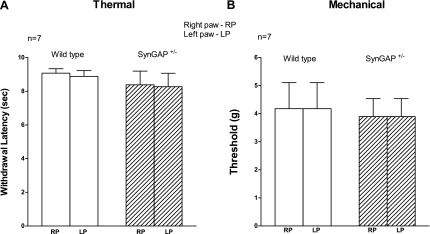
Although reduced SynGAP expression augments capsaicin-evoked release of iCGRP from sensory neurons, the question remains as to whether this enhanced responsiveness results in altered nociception. Thus behavioral responses to thermal and mechanical stimuli in WT and SynGAP+/− littermates were tested. As can be seen in Fig. 5, there were no differences between WT and SynGAP+/− mice in either basal thermal or mechanical nociceptive behaviors, suggesting that the reduction in SynGAP does not alter acute nociceptive responses.
Fig. 5.
Thermal and mechanical nociceptive behaviors in WT and SynGAP+/− littermates. A: basal paw-withdrawal latency to radiant heat was measured in both the right hindpaw (RP) and the left hindpaw (LP) in WT or SynGAP+/− mice as indicated. Data are presented as the mean ± SEM of withdrawal latency in seconds for 7 animals of each genotype. B: basal paw-withdrawal response to mechanical stimulus (Von Frey filaments) was measured in both the RP and the LP in WT or SynGAP+/− mice, as indicated. Data are presented as the mean ± SEM of the threshold for withdrawal in grams of force for 7 animals of each genotype. There were no significant differences between SynGAP+/− and WT mice responses using Student's t-test.
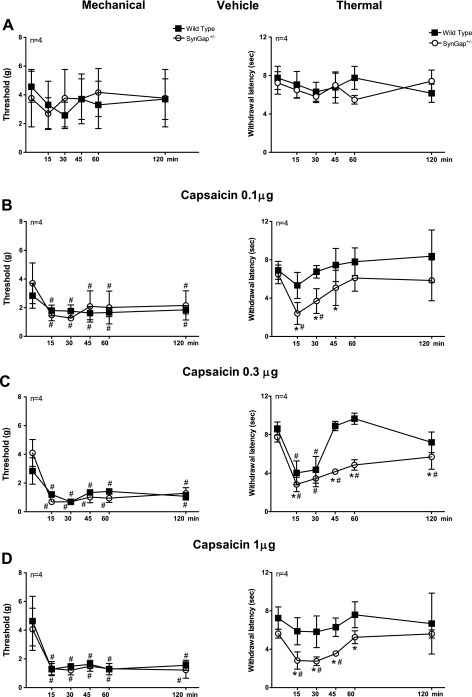
Intraplantar injection of vehicle (0.5% MPL v/v) did not significantly alter thermal- or mechanical-withdrawal responses in either genotype (Fig. 6A). Injection of 0.1, 0.3, or 1.0 μg of capsaicin reduced the threshold for mechanical stimulation in both WT and SynGAP+/− mice equally, and this reduction was maintained for 120 min following the injection (Fig. 6, B–D). There was no significant difference in the capsaicin-induced mechanical hypernociception between WT and SynGAP+/− mice at any of the capsaicin doses tested. Capsaicin was used as a stimulus in these behavioral investigations, since we had observed an increase of iCGRP release with capsaicin and an increase in TRPV1 expression in the SynGAP+/− mice (Figs. 2 and 4). In contrast to mechanical stimulation, SynGAP+/− mice showed an enhanced hypernociceptive response to thermal stimuli after capsaicin injection compared with WT mice. Intraplantar injection of 0.1 μg of capsaicin in WT mice had no effect on thermal latency (Fig. 6B), whereas a significant decrease in the paw-withdrawal latency was observed in SynGAP+/− mice injected with this dose. Injection of 0.3 μg of capsaicin caused the same reduction in thermal latency in WT and SynGAP+/− mice at 15 and 30 min after injection, but the decrease in the withdrawal threshold was maintained for at least 60 min in SynGAP+/− mice, whereas the hypernociception resolved after 30 min in WT mice (Fig. 6C). At 1 μg, capsaicin produced sustained thermal hypernociception in SynGAP+/− but not in WT mice (Fig. 6D). These results clearly demonstrate that decreased SynGAP expression leads to increased capsaicin sensitivity, resulting in enhanced capsaicin-induced thermal hypernociception.
Fig. 6.
Mechanical and thermal hypernociception induced by intraplantar injection of capsaicin in WT and SynGAP+/− mice. Time course of mechanical nociceptive responses (left panels) or thermal nociceptive responses (right panels) in vehicle-treated (A) or capsaicin-treated (B–D) WT (■) or SynGAP+/− (○) mice. Each point is the mean ± SEM for withdrawal in grams of force (left panels) or threshold for withdrawal latency in seconds (right panels) of 4 animals of each genotype/group. *Statistically significant difference (P < 0.05) between genotypes; #statistically significant difference (p <0.05) between before and after intraplantar capsaicin injection using 2-way repeated-measures ANOVA, followed by Bonferroni post-hoc analysis.
DISCUSSION
Until now, SynGAP, a neuron-specific Ras/Rap-GAP first described in the brain (Chen et al. 1998; Kim et al. 1998), has been implicated only in synaptic transmission occurring in the CNS (Kim et al. 2003; Komiyama et al. 2002; Rumbaugh et al. 2006). Our data are the first to show that SynGAP is expressed in sensory neurons of the PNS. Additionally, we demonstrate that SynGAP plays a role in regulating capsaicin-induced peripheral sensory neuron sensitization, as indicated by an augmentation of release of iCGRP and thermal hypernociception in SynGAP+/− mice.
Despite the increase in capsaicin-stimulated release of iCGRP from SynGAP+/− sensory neurons compared with WT neurons, there was no difference in intrinsic excitability of the neurons from the two genotypes, as shown by the similar electrical properties and responses to ramps of depolarizing current. Consistent with these observations was the lack of a difference in iCGRP release evoked by high extracellular potassium or in baseline behavioral responses to thermal or mechanical stimulation. These results are consistent with studies of CNS neurons, where Komiyama et al. (2002) and Kim et al. (2003) found no alteration in basal pre- or postsynaptic components of synaptic transmission from CA1 hippocampus neurons in tissue slices from SynGAP+/− mice compared with those from WT mice. In addition, there was no difference in numbers of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid or NMDA receptors in hippocampal tissue from either genotype. Together, these observations demonstrate that a significant reduction in the expression of SynGAP and the downstream consequences of this altered expression do not alter the excitable membrane properties of neurons in either the CNS or PNS.
The basal behavioral response to mechanical and thermal stimuli was similar in the SynGAP+/− and WT mice, suggesting that decreased SynGAP expression does not alter basic sensory function. With the use of a separately generated SynGAP+/− mouse, Muhia et al. (2010) also demonstrated no difference in the withdrawal latency to thermal stimulation with altered SynGAP expression. Although SynGAP+/− mice, similar to the strain we used, exhibited an increase in spontaneous, seemly purposeless, open-field activity and an increased startle response, there was no evidence of gross sensory or motor dysfunction reported in these animals (Guo et al. 2009).
Capsaicin can evoke spontaneous pain and thermal and mechanical hyperalgesia with well-defined peripheral and central components in both humans and animals (Gilchrist et al. 1996; Simone et al. 1989). There is a consensus that primary thermal hyperalgesia, which occurs at the site of capsaicin injection, is caused by peripheral nociceptor sensitization, whereas secondary mechanical hyperalgesia is a result of both peripheral and central sensitization in the dorsal horn of the spinal cord (Gilchrist et al. 1996; Koltzenburg et al. 1992; LaMotte et al. 1991; Simone et al. 1989). We show that capsaicin induces change in nociceptive responses to a thermal or mechanical stimuli and that the duration of capsaicin-induced mechanical hypernociception was longer than that of thermal hypernociception. This time course is similar to that previously observed in rats (Gilchrist et al. 1996). Capsaicin-induced thermal hypernociception in SynGAP+/− mice occurred at lower doses of capsaicin and had a longer duration than WT littermates. In addition, expression of TRPV1, the capsaicin receptor, was threefold higher in DRG preparations from SynGAP+/− mice than WT littermates. This suggests that the increased responses to capsaicin observed in SynGAP+/− tissue were secondary to increased receptor expression. Activation of Ras and downstream signaling cascades activated by Ras and Rap1 can alter the expression of TRPV1 in sensory neurons. Bron et al. (2003) demonstrated that exposure to NGF caused an increase in expression of TRPV1 in sensory neurons, and this response was dependent on MEK activation. Moreover, expression of a dominant-negative form of Ras also prevented NGF-induced increases in TRPV1 expression in these cells. MEK activity was also necessary for NGF-induced increases in capsaicin current (Zhuang et al. 2004) and TNF-α-induced increases in TRPV1 receptor expression in DRG neurons (Hensellek et al. 2007). Thus decreased SynGAP expression leading to increased Ras/Rap1 signaling and increased levels of p-ERK, as we observed, can explain the increased TRPV1 expression and capsaicin-mediated responses that we demonstrated in the SynGAP+/− mice. Whether it is elevated ERK activation itself, a downstream effector of ERK, parallel pathways activated by Ras/Rap1, or some combination of these pathways that results in increased TRPV1 expression is currently unknown.
Enhanced Ras signaling, elevated p-ERK, and other downstream effectors of the Ras cascade can increase peripheral sensitization. For example, Zhuang et al. (2004) demonstrated that both MEK and phosphatidylinositol 3-kinase activity are important in NGF-mediated increases in thermal hypernociception. Dai et al. (2002) showed that after intraplantar injection of capsaicin, there is an activation of ERK pathways at the nerve terminals in the skin and to a lesser extent, in the neuron cell bodies of the DRG. In these experiments, capsaicin-induced thermal hypernociception was diminished by pretreatment with a MEK inhibitor, U0126, into the paw prior to capsaicin injection. In contrast, pretreatment with the MEK inhibitor had no effect on capsaicin-induced secondary mechanical allodynia. Since primary hyperalgesia is mediated by sensitization of primary afferent neurons (peripheral sensitization), whereas secondary hyperalgesia involves both peripheral and central sensitization mechanisms (Raja et al. 1999), it was suggested that changes in ERK signaling in the nerve terminals were important in the development of peripheral sensitization but not in the development of central sensitization, an essential component for the development of secondary hyperalgesia. We also observed a change in capsaicin-induced thermal sensitivity in the SynGAP+/− mice with enhanced p-ERK levels but no change in mechanical responses to capsaicin between the genotypes. One explanation for this is that the increased Ras/Rap1 signaling in the SynGAP+/− mice results in increased peripheral sensitization but does not alter central sensitization processes. Interestingly, we did not observe any changes in capsaicin-induced mechanical hypernociception in the SynGAP+/− mice compared with WT littermates. Again, because capsaicin-induced mechanical allodynia requires central sensitization in addition to peripheral sensitization (Raja et al. 1999), our results may suggest that reduced SynGAP expression does not alter capsaicin-induced central sensitization.
In contrast to SynGAP+/− mice, sensory neurons from mice with a heterozygous mutation of neurofibromatosis-1 (Nf1+/−), encoding the Ras-GAP neurofibromin, do exhibit an increase in excitability to a ramp of depolarizing current (Wang et al. 2005). In these neurons from Nf1+/− mice with reduced expression of neurofibromin, the increased excitability can be explained by a significant increase in sodium current density compared with WT neurons (Wang et al. 2010). The stimulus-evoked release of iCGRP is also enhanced in sensory neurons isolated from Nf1+/− mice compared with that from WT mice (Hingtgen et al. 2006). However, the Nf1+/− sensory neurons exhibit increased iCGRP release for stimulation by either high extracellular potassium or capsaicin. One explanation for the different consequences seen with reduction of SynGAP and neurofibromin may be based on the selectivity of the two GAPs for more than one small G-protein. Although both neurofibromin and SynGAP are GAPs for Ras, SynGAP also can regulate the activity of two other small G-proteins: Rap1 and Rab (Krapivinsky et al. 2004; Tomoda et al. 2004). The gene for Rap1 encodes a small G-protein structurally related to Ras (Reuther and Der 2000). Like Ras, Rap1 serves as a molecular switch that couples extracellular signals, such as growth factor receptor activation and heterotrimeric G-protein activation, to various cellular responses (Bos et al. 2001). Interestingly, activation of Rap1 and Ras can have different consequences, even when activated by the same extracellular signal. For example, NGF initiates both Rap1-GTP-dependent and Ras-GTP-dependent activation of MAPKs in PC12 cells (York et al. 1998). However, the growth factor-induced activation of Rap1- and Ras-dependent signaling is regulated such that transient exposure to a growth factor acts preferentially through Ras-GTP-dependent mechanisms, and more sustained exposure to a growth factor signals through Rap1-GTP-dependent mechanisms. In addition, glutaminergic synaptic transmission in hippocampal slices can be enhanced by Ras activation but inhibited by Rap activation (Imamura et al. 2003; Zhu et al. 2002). In a neuronal cell line, introduction of a constitutively active form of Ras increased AP generation, whereas introduction of a constitutively active form of Rap1 decreased AP formation (Imamura et al. 2004). This difference was explained by modulation of voltage-gated sodium currents. Thus the lack of increase in excitability in sensory neurons from SynGAP+/− mice may be a result of the combination of activation of both Ras and Rap1 in contrast to a more Ras-dominated activation in the Nf1+/− sensory neurons.
Although the understanding of the role of GAP in sensitization of sensory neurons is only beginning, our results clearly demonstrate the importance of SynGAP in the regulation of sensory neuron small G-protein pathways and peripheral sensitization. Since Ras can be activated by growth factors and cytokines critical in injury and inflammation, these results provide an intriguing and novel pathway in pain and peripheral sensitization worthy of additional exploration.
GRANTS
This research was supported in part by the National Institute of Neurological Disorders and Stroke R01 NS051668 (C. M. Hingtgen) and R01 NS048565 (M. R. Vasko).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. Richard Huganir for generously providing the original SynGAP+/− mice for breeding. We also thank Shannon Roy for her assistance in breeding the mice necessary for these investigations and Eric L. Thompson and Dr. Jill Fehrenbacher for technical assistance.
REFERENCES
- Bos JL, de Rooij J, Reedquist KA. Rap1 signaling: adhering to new models. Nat Rev Mol Cell Biol 2: 369–377, 2001 [DOI] [PubMed] [Google Scholar]
- Bron R, Klesse LJ, Shah K, Parada LF, Winter J. Activation of Ras is necessary and sufficient for upregulation of vanilloid receptor type 1 in sensory neurons by neurotrophic factors. Mol Cell Neurosci 22: 118–132, 2003 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55–63, 1994 [DOI] [PubMed] [Google Scholar]
- Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron 20: 895–904, 1998 [DOI] [PubMed] [Google Scholar]
- Chen JJ, Barber LA, Dymshitz J, Vasko MR. Peptidase inhibitors improve recovery of substance P and calcitonin gene-related peptide release from rat spinal cord slices. Peptides 17: 31–37, 1996 [DOI] [PubMed] [Google Scholar]
- Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci 22: 7737–7745, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan S, Shannon KM, Bollag G. GTPase activating proteins: critical regulators of intracellular signaling. Biochim Biophys Acta 1602: 23–45, 2002 [DOI] [PubMed] [Google Scholar]
- Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain 67: 179–188, 1996 [DOI] [PubMed] [Google Scholar]
- Guo X, Hamilton PJ, Reish NJ, Sweatt JD, Miller CA, Rumbaugh G. Reduced expression of the NMDA receptor-interacting protein SynGAP causes behavioral abnormalities that model symptoms of schizophrenia. Neuropsychopharmacology 34: 1659–1672, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32: 77–88, 1988 [DOI] [PubMed] [Google Scholar]
- Hensellek S, Brell P, Schaible HG, Brauer R, Segond von Banchet G. The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci 36: 381–391, 2007 [DOI] [PubMed] [Google Scholar]
- Hingtgen CM, Roy SL, Clapp DW. Stimulus-evoked release of neuropeptides is enhanced in sensory neurons from mice with a heterozygous mutation of the Nf1 gene. Neuroscience 137: 637–645, 2006 [DOI] [PubMed] [Google Scholar]
- Hingtgen CM, Vasko MR. Prostacyclin enhances the evoked-release of substance P and calcitonin gene-related peptide from rat sensory neurons. Brain Res 655: 51–60, 1994 [DOI] [PubMed] [Google Scholar]
- Imamura Y, Matsumoto N, Kondo S, Kitayama H, Noda M. Possible involvement of Rap1 and Ras in glutamatergic synaptic transmission. Neuroreport 14: 1203–1207, 2003 [DOI] [PubMed] [Google Scholar]
- Imamura Y, Matsumoto N, Kondo S, Kitayama H, Noda M. Effects of Ras and Rap1 on electrical excitability of differentiated NG108–15 cells. Neuroscience 127: 973–981, 2004 [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 2: 1114–1119, 1999 [DOI] [PubMed] [Google Scholar]
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis 8: 1–10, 2001 [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol 9: 517–531, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee HK, Takamiya K, Huganir RL. The role of synaptic GTPase-activating protein in neuronal development and synaptic plasticity. J Neurosci 23: 1119–1124, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Liao D, Lau LF, Huganir RL. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron 20: 683–691, 1998 [DOI] [PubMed] [Google Scholar]
- Knuesel I, Elliott A, Chen HJ, Mansuy IM, Kennedy MB. A role for SynGAP in regulating neuronal apoptosis. Eur J Neurosci 21: 611–621, 2005 [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Lundberg LE, Torebjork HE. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain 51: 207–219, 1992 [DOI] [PubMed] [Google Scholar]
- Komiyama NH, Watabe AM, Carlisle HJ, Porter K, Charlesworth P, Monti J, Strathdee DJ, O'Carroll CM, Martin SJ, Morris RG, O'Dell TJ, Grant SG. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci 22: 9721–9732, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Medina I, Krapivinsky L, Gapon S, Clapham DE. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron 43: 563–574, 2004 [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol 66: 190–211, 1991 [DOI] [PubMed] [Google Scholar]
- Muhia M, Yee BK, Feldon J, Markopolous F, Knuesel I. Disruption of hippocampus-regulated behavioural and cognitive processes by heterozygous constitutive deletion of SynGAP. Eur J Neurosci 31: 529–543, 2010 [DOI] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol Interv 7: 26–41, 2007 [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Dai Y, Tachibana T, Fukuoka T, Tokunaga A, Yoshikawa H, Noguchi K. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J Neurosci 23: 4117–4126, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja SN, Meyer RA, Ringkamp M, Campbell JN. Peripheral neural mechanisms of nociception. In: Textbook of Pain, edited by Wall PD, Melzack R. Oxford, UK: Churchill Livingstone, 1999 [Google Scholar]
- Reuther GW, Der CJ. The Ras branch of small GTPases: Ras family members don't fall far from the tree. Curr Opin Cell Biol 12: 157–165, 2000 [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Adams JP, Kim JH, Huganir RL. SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proc Natl Acad Sci USA 103: 4344–4351, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffzek K, Ahmadian MR, Wittinghofer A. GTPase-activating proteins: helping hands to complement an active site. Trends Biochem Sci 23: 257–262, 1998 [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain 38: 99–107, 1989 [DOI] [PubMed] [Google Scholar]
- Tomoda T, Kim JH, Zhan C, Hatten ME. Role of Unc51.1 and its binding partners in CNS axon outgrowth. Genes Dev 18: 541–558, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasko M, Campbell WB, Waite KJ. Prostaglandin E2, enhances bradykinin-stimulated release of neuropeptides from rat sensory neurons in culture. J Neurosci 14: 4987–4997, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Duan JH, Hingtgen CM, Nicol GD. Augmented sodium currents contribute to the enhanced excitability of small diameter capsaicin-sensitive sensory neurons isolated from Nf1+/− mice. J Neurophysiol 103: 2085–2094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nicol GD, Clapp DW, Hingtgen CM. Sensory neurons from Nf1 haploinsufficient mice exhibit increased excitability. J Neurophysiol 94: 3670–3676, 2005 [DOI] [PubMed] [Google Scholar]
- York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJS. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 392: 622–626, 1998 [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell 110: 443–455, 2002 [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci 24: 8300–8309, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]