Abstract
Neurons exhibiting on and off responses with different frequency tuning have previously been described in the primary auditory cortex (A1) of anesthetized and awake animals, but it is unknown whether other tuning properties, including sensitivity to binaural localization cues, also differ between on and off responses. We measured the sensitivity of A1 neurons in anesthetized ferrets to 1) interaural level differences (ILDs), using unmodulated broadband noise with varying ILDs and average binaural levels, and 2) interaural time delays (ITDs), using sinusoidally amplitude-modulated broadband noise with varying envelope ITDs. We also assessed fine-structure ITD sensitivity and frequency tuning, using pure-tone stimuli. Neurons most commonly responded to stimulus onset only, but purely off responses and on-off responses were also recorded. Of the units exhibiting significant binaural sensitivity nearly one-quarter showed binaural sensitivity in both on and off responses, but in almost all (∼97%) of these units the binaural tuning of the on responses differed significantly from that seen in the off responses. Moreover, averaged, normalized ILD and ITD tuning curves calculated from all units showing significant sensitivity to binaural cues indicated that on and off responses displayed different sensitivity patterns across the population. A principal component analysis of ITD response functions suggested a continuous cortical distribution of binaural sensitivity, rather than discrete response classes. Rather than reflecting a release from inhibition without any functional significance, we propose that binaural off responses may be important to cortical encoding of sound-source location.
Keywords: auditory cortex, binaural interactions, interaural time delay sensitivity, interaural level difference sensitivity
the acoustic cues for sound localization comprise interaural level differences (ILDs) and spectral cues at high frequencies and interaural time delays (ITDs) at lower frequencies. The importance of a functioning primary auditory cortex (A1) in sound localization has been demonstrated through lesion experiments in a range of species (e.g., Heffner and Heffner 1990; Jenkins and Merzenich 1984; Kavanagh and Kelly 1987; Nodal et al. 2010a). Several studies have investigated the manner in which cortical neurons encode spatial location (Brugge et al. 1994; Campbell et al. 2006; Fitzpatrick and Kuwada 2001; Middlebrooks et al. 1994; Mrsic-Flogel et al. 2005; Recanzone 2000b). Although ferrets have been used in a number of behavioral and physiological studies of spatial hearing, surprisingly little is currently known about neuronal responses to ITDs at any level of the auditory system in this species.
Of the studies that have investigated spatial or ILD sensitivity in ferret A1, the majority have concentrated on responses to the onset of the stimulus alone (Campbell et al. 2006; Mrsic-Flogel et al. 2001). On responses are the dominant neuronal response type recorded in A1 under barbiturate anesthesia (Phillips and Hall 1990), whereas neurons with more complex temporal response patterns have been described in ketamine-anesthetized (Volkov and Galazjuk 1991), halothane-anesthetized (Moshitch et al. 2006), and awake (Wang et al. 2005) animals. Moshitch and colleagues (2006) showed that neural responses to pure tones can carry information about stimulus identity up to ∼300 ms following stimulus offset in halothane-anesthetized cats. More recently, Campbell et al. (2010) found that ILD information is retained over a similar period in ketamine-anesthetized ferrets. By presenting stimuli from a spatially fixed free-field speaker, Qin et al. (2007) reported that the pure-tone sensitivity of A1 neurons in cats often changes between stimulus onset and offset. It is not known, however, whether cortical sensitivity to auditory spatial cues changes over the course of the response in a similar fashion.
A recent development in our laboratory has been to investigate binaural sensitivity in ferrets with closed-field behavioral measures (Nodal et al. 2010b), in addition to more established techniques of free-field sound localization (Parsons et al. 1999) and signal-in-noise detection (Hine et al. 1994). Furthermore, the first behavioral model of bilateral cochlear implantation in a nonhuman species has recently been developed in the ferret to investigate the effects of hearing loss, and chronic intracochlear electrical stimulation, on binaural hearing (Hartley et al. 2010). Together these studies provide us with additional impetus to learn how binaural cues are encoded in the central auditory pathway of normally hearing animals.
The aim of the present study was to investigate neural response patterns to different sound localization cues in ferret A1. The emphasis of the study was investigation of the convergence of cortical spatial sensitivity to ILDs, ITDs, and the frequency content of the stimuli following the onset and offset of binaural stimulation, and to characterize any potential binaural response classes.
METHODS
Animal Preparation
Seven adult pigmented ferrets (Mustela putorius) were used in this study, and all animal procedures were approved by a local ethics committee and licensed by the UK Home Office. Otoscopy was performed prior to the experiment to ensure that both external ear canals were patent and disease free. Anesthesia was induced by intramuscular administration of medetomidine hydrochloride (Domitor, 0.08 mg/kg; Pfizer). At the time of induction, an intramuscular injection of atropine sulfate (0.06 mg/kg; C-Vet Veterinary Products) was given to reduce the risk of cardiac arrhythmias and to limit airway secretions. After induction, a 24-gauge cannula was inserted into the cephalic vein and anesthesia was maintained with a continuous infusion of a mixture of medetomidine hydrochloride (0.02 mg·kg−1·h−1) and ketamine (Ketaset, 5 mg·kg−1·h−1; Fort Dodge Animal Health) in 5% glucose-saline solution. To minimize cerebral edema and bronchial secretions, the infusion was supplemented with 0.5 mg·kg−1·h−1 dexamethasone (Dexadreson; Intervet UK) and 0.06 mg·kg−1·h−1 atropine sulfate, respectively. The animal was intubated with a 3-mm internal diameter, uncuffed, endotracheal tube (Portex, Smiths Medical International) inserted under direct laryngoscopy. Subsequently, the animal was ventilated with an oxygen-air mix, and its body temperature, end-tidal CO2, and electrocardiogram were monitored throughout the experiment. The animal's head was fixed in a stereotaxic frame with blunt ear bars, and the skull was exposed. A steel head bar was attached to the skull with stainless steel screws and dental cement, and, subsequently, the stereotaxic frame was removed. A craniotomy was performed to expose the left auditory cortex, the dura was removed, and cortical motion was reduced with a thin layer of 1.5% agar in saline.
Stimuli
All stimuli were generated with Tucker-Davis Technologies System III hardware and Brainware and Real Time Processor Visual Design Studio (RPvdsEx) software (50-kHz sampling rate). Subsequently, they were presented binaurally over earphones (Panasonic RP-HV297) coupled to otoscope specula that were inserted into each ear canal. Before each experiment, the transfer function of the earphones was canceled from the stimulus by using an inverse filter to ensure the frequency response of the drivers was flat from 0.5 to 25 kHz (±2 dB). Closed-field calibration was carried out with a ⅛-in. condenser microphone (type 4138, Brüel & Kjaer UK).
Characterization stimuli.
Diotic broadband noise (70-dB SPL; 0.05- to 30-kHz bandwidth; 100-ms duration with a 5-ms cosine-squared rise-fall time) was used as a search stimulus in order to establish whether a unit was acoustically responsive. Subsequently, the frequency tuning of each responsive unit was characterized with diotically presented pure tones (100-ms duration with 10-ms rise-fall times) over a range of frequencies (0.4–22 kHz in half-octave steps) and levels (60- to 80-dB SPL in 10-dB steps). The best frequency (BF) of a unit was defined as the tone frequency that elicited the largest number of spikes within a 500-ms recording window after the onset of the stimulus. ILD and envelope ITD (eITD) sensitivity was assessed in all driven units with stimuli that are described in detail below. All units with a BF of ≤2.5 kHz were assessed for fine-structure ITD (fITD) sensitivity, in addition to ILD and eITD sensitivity.
ILD stimuli.
ILD sensitivity was assessed with broadband noise. Specifically, ILD sensitivity (−40 to +40 dB in 4-dB steps) was measured with unmodulated broadband noise presented to both ears over a range of average binaural levels (40- to 70-dB SPL in 10-dB steps). Since ferrets rarely experience ILDs >35 dB while localizing sounds (Parsons et al. 1999), ILDs were predominantly presented within the physiological range. Although it might have been interesting to include ILDs outside the physiological range, it was important to limit the maximum ILD (±40 dB) to below the cross talk level of our sound delivery system (Nodal et al. 2010b). Negative ILDs indicated that the sound was louder in the ear contralateral to the recording site.
ITD stimuli.
ITD sensitivity to the fine structure of pure tones is restricted to frequencies below ∼1.3 kHz in human psychophysical studies (Zwislocki and Feldman 1956) and ∼2.5 kHz in physiological studies in cats (Jackson et al. 1996; Reale and Brugge 1990). In contrast, ITD sensitivity to envelopes of high-frequency signals is limited to below 200 Hz in human psychophysical studies (Bernstein and Trahiotis 2002) and ∼600 Hz in animal physiological studies (Griffin et al. 2005; Joris and Yin 2007). In the guinea pig, an animal that has a head size similar to that of the ferret, it has been shown that neurons often respond most strongly to ITDs that occur outside the normal physiological range of ITDs (McAlpine et al. 1996, 2001). Therefore, the values of ITDs tested in this study were chosen to extend beyond the physiological range of ITDs that a ferret would realistically encounter within its free-field acoustic environment. Acoustic measurements from microphones placed in the external auditory meatus suggest that the physiological range of ITDs experienced by a ferret will be approximately ±200–300 μs (Schnupp et al. 2003). In the present study we used a range of ITDs that spanned ±1 ms in 0.1-ms steps. The maximal ITDs used in the present study, namely ±1 ms, correspond to an interaural phase difference of ±0.15, 0.3, 0.45, and 0.6 cycles at 150, 300, 450, and 600 Hz, respectively.
Sensitivity to eITDs was measured with sinusoidal amplitude-modulated (SAM) broadband noise presented to both ears over a range of modulation frequencies (100% modulated; 70-dB SPL; 100- to 500-ms duration). To evaluate eITD sensitivity independent of fITD sensitivity, identical noise carriers were constructed for each ear that were modulated by sinusoids with an interaural delay (−1 to +1 ms in 0.1-ms steps). The stimuli were gated with an identical interaural delay to the modulator and 5-ms cosine-squared rise-fall times in each ear. Consequently, the envelope of the stimulus contained onset, ongoing, and offset ITDs, while the fine structure of the carrier remained in phase (at zero delay) between the ears. A positive ITD was generated by delaying the stimulus in the ear ipsilateral to the recording site (left) and advancing the stimulus in the contralateral ear. A negative ITD was generated by delaying the stimulus in the ear contralateral to the recording site (right) and advancing the stimulus in the ipsilateral ear. The range of modulation frequencies tested in the present study (150–600 Hz in 150-Hz steps) was designed to include frequencies that spanned the range to which a previous study showed that neural sensitivity to envelope ITDs was maximal (Griffin et al. 2005).
For low-frequency units (BF ≤2.5 kHz), fITD sensitivity (−1 to +1 ms in 0.1-ms steps) was measured with dichotic pure tones (70-dB SPL; 100- to 500-ms duration with 5-ms cosine-squared rise-fall times) over a range of frequencies (4 frequencies distributed in 50-Hz steps around the BF of the unit). Although the chosen frequencies were all close to BF, this ensured that the responsiveness of the unit was maintained across all stimulus conditions. ITDs were generated by delaying the entire stimulus in one ear while advancing the waveform in the other ear by an equal amount. Thus the dichotic stimulus contained onset, ongoing, and offset ITDs.
For each binaural condition (ILD, eITD, and fITD), there were 84 stimulus parameter combinations that were presented 15 times each in a pseudorandom order. The basic protocol required ∼45 min for low-frequency units and slightly less time for high-frequency units since fITD measurements were omitted. For most units the protocol could be completed in its entirety without a drift in the firing rate of the recordings over time.
For the majority of recordings, stimuli were 100-ms duration gated with 5-ms rise-fall times. To determine whether later responses were related to the offset of the stimulus, in a subset of units ILD, eITD, and fITD sensitivity was assessed in response to 500-, 400-, 200-, and 20-ms-duration stimuli. For stimuli of ≤200-ms duration, stimuli were presented at a rate of 1 every 500 ms, and for durations of ≥400 ms, stimuli were presented every 1 s.
Electrophysiological Recordings
All electrophysiological recordings were carried out in a sound-attenuated chamber (Industrial Acoustics). Extracellular single-unit and small multiunit cluster activity was recorded with 16-channel silicon probes (NeuroNexus Technologies). The silicon probes consisted of a single shank with 16 active recording sites (intersite separation 100 μm; 1.5 mm between apical and basal recording sites). The ferret auditory cortex is ∼1.7–2 mm in depth (Dahmen et al. 2008). Thus, when the proximal electrode of the silicon probe was just inserted, perpendicular to the cortical surface, recordings could be made from cortical layers I to V simultaneously. All recordings were band-pass filtered (0.5–5 kHz), amplified, and digitized at 25 kHz. Stimulus generation and data acquisition were synchronized with Brainware software (Tucker-Davis Technologies). Data were acquired in 0.5- to 1-s sweeps that were triggered by the onset of the stimulus. Any recorded event with a magnitude of 2.5 times the root mean squared amplitude of the recorded signal was considered to be a potential spike. The latency and shapes of all potential spikes were stored for off-line analysis. A1 was targeted with sulcal landmarks according to the criteria of Bizley et al. (2005). Ferret A1 is tonotopically organized, with high-frequency regions represented mainly within the dorsal tip of the middle ectosylvian gyrus (MEG) and low frequencies more ventrally (Bizley et al. 2005). Based on anatomic landmarks, we predominantly sampled from ventral regions of the MEG and, for each animal, ∼10–40 evenly spaced recording sites were selected.
Data Analysis
Spike sorting.
Spike sorting algorithms were based on those used in previous studies within our laboratory (Campbell et al. 2006). Spikes were sorted off-line with a k-means clustering algorithm incorporated into Brainware. Specifically, clusters were chosen on the basis of spike shape. Subsequently, a test of the refractory period in the autocorrelation histogram was used to assess whether the chosen cluster was more likely to be a single unit or a multiunit. Any cluster containing <1% of spikes with an interspike interval of <1.5 ms was classed as a single unit, while the remaining clusters were classed as small multiunits. These parameters were somewhat arbitrary; we cannot rule out the possibility that units classified as single units might have included a small proportion of multiunit responses. Spike times were then exported into Matlab 7.0.1 (The MathWorks) for further analysis.
Response period.
Initially, for each recording a peristimulus time histogram (PSTH), with a 1-ms bin width, was constructed by counting the number of spikes evoked during each trial. Similar to previous studies in awake cats (Qin et al. 2007) and ketamine-anaesthetized rats (Scholl et al. 2010), excitatory responses in A1 were classified based on the discharge patterns, with particular attention to responses around the onset and the offset of the stimulus. Subsequently, spectro-temporal and binaural-temporal response functions were constructed by calculating the spike rate in 5-ms bins following the stimulus onset in response to each stimulus condition, averaging across 15 repetitions. Spikes counts were averaged with a 70-ms duration window positioned between 5 and 75 ms after the onset or the offset of the stimulus. These windows were chosen after initial analysis of the peak latency of on and off responses across our population of neurons (Fig. 1). The background or spontaneous spike rate for each recording was calculated from the PSTH, by averaging the spikes occurring between 5 and 75 ms before the stimulus onset. With a Poisson cumulative distribution function, an excitatory response in the onset and offset periods was defined as “driven” if the spike rate in the response period was greater than that of the spike rate in a spontaneous window with a probability of ≥0.99. In anesthetized rats, Scholl and colleagues (2010) measured spike rates using a fixed window following the onset and offset of the signal and found that varying the duration of the spike-averaging window from 50 to 100 ms did not significantly alter their results. Likewise, in the present study, we found similar results after reducing our spike averaging window from 70 to 35 ms (data not shown).
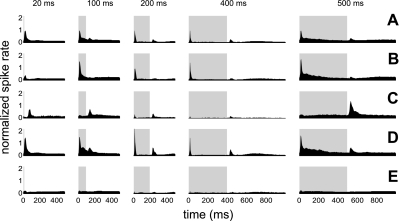
Fig. 1.
Average peristimulus time histogram (PSTH) plotted for each stimulus duration for all units (A), for units with a significant on response alone (B), for units with a significant off response alone (C), for units with a significant on and off response (D), and for unresponsive units (E). The average PSTH plotted for all units was given a maximum spike rate of 1 (A). All other spike rates were plotted relative to this value (B–E). The stimulus duration is represented by the light gray bar.
Binaural sensitivity.
In units with a significant on and/or off response, binaural sensitivity (ILD, eITD, and/or fITD sensitivity) was assessed by determining whether Poisson regression models of up to 4th order fitted the data significantly better than a null model that assumed no effect of stimulus. A likelihood ratio test was performed to determine whether the Poisson regression model fitted the data significantly better than the null model at P < 0.05. In other words, the observed spike counts in any one trial y as a function of stimulus parameter x were fit as polynomials of the form
with the Matlab generalized linear modeling function “glmfit” where b0 … b4 are free parameters of the model. Such polynomials can approximate many of the nonlinearities that might be expected in physiological stimulus-response relationships, such as thresholding, saturation, and non-monotonicity. Subsequently, they make few assumptions about the shape and dependence of a neuron's response on stimulus parameter x. The deviance of the model Dm was then compared against the deviance D0 of the null model
i.e., a model that assumes that the firing is entirely independent of stimulus parameter x, and merely varies randomly around some spontaneous rate b0, exploiting the fact that the difference in the deviances D0 − Dm is equivalent to minus twice the difference in log-likelihoods of the two models given the data, and is therefore approximately χ2-distributed with a number of degrees of freedom equal to the order of the fitted polynomial.
Of those units that showed significant binaural sensitivity within both the on and off response periods, a bootstrap test was used to test whether the pattern of responses as a function of stimulus parameters seen in the on period differed significantly from that seen in the off period. The bootstrap test determined whether the difference in on and off response patterns, quantified by their Euclidean distance, was larger than would be expected by chance, where the differences expected by chance were estimated by randomly allocating observed spike counts among stimulus conditions and onset and offset periods. The original observed difference between onset and offset was deemed significant if it exceeded the difference obtained in the randomly resampled data in at least 95% of 1,000 cases.
Clustering analyses.
Although some authors have previously classified cortical responses to binaural stimulation into distinct binaural response classes, a recent study indicates that cortical ILD tuning curves may fall along a continuum, rather than forming naturally discrete classes (Campbell et al. 2006). Here we similarly investigated the distribution of observed binaural response profiles for evidence of naturally occurring classes, using hierarchical clustering methods described by Martinez and Martinez (2002) and principal component analysis (PCA). Briefly, the response functions generated from on responses to eITD stimuli were normalized (z-scored so that they had a mean of 0 and a standard deviation of 1) and arranged into an N × S matrix of N units recorded at S = 84 stimulus combinations. A PCA of this matrix was then carried out. The first component accounts for the greatest amount of variance in spike count as a function of stimulus parameter, with each successive component accounting for progressively less variance (see results). Subsequently, clustering-based analyses were performed both on the raw data and on their principal component representations, in an attempt to classify cortical ITD responses into distinct response classes.
RESULTS
The results are based on a total of 546 acoustically responsive units (of which 75% were classified as single units based on our criteria) sampled from the left auditory cortex of seven adult ferrets. ILD and eITD stimuli of 100-ms duration were presented in 74% of recordings (405 units), regardless of the BF of the unit. fITD stimuli of 100-ms duration were presented only to units with a BF of ≤2.5 kHz (295 units). Additional ILD, eITD, and fITD stimuli of 20- and 500-ms duration were presented in 50% of all recordings. Because of experimental time constraints, 200- and 400-ms stimuli were presented less frequently (9% of recordings).
Timescale of Cortical Responses
During the initial analysis, the average PSTH was plotted for responses to all stimuli of 20-, 100-, 200-, 400-, and 500-ms duration (Fig. 1A). Functions were smoothed with a 3-ms moving average function. In response to stimuli of >100-ms duration, the mean PSTH across all units analyzed revealed excitatory responses that occurred predominantly after the onset and the offset of the stimulus (Fig. 1A). On average, distinct on and off responses were not seen for stimuli of 20-ms duration. Consequently, responses to 20-ms stimuli were not analyzed further. A threshold was used to classify responses that had a significant peak in the PSTH above the spontaneous firing rate at any given recording location within the onset- or offset-response window. This analysis revealed that, based on this criterion, 54.9% of recording locations yielded acoustically responsive units. Of these responsive units (n = 546), ∼59% had a significant on response alone (Fig. 1B), 13% had a significant off response alone (Fig. 1C), and 28% of cells had significant on and off responses (Fig. 1D). In just less than half of all recording locations there was no significant on or off response to the stimulus (Fig. 1E).
In most cases the group data were representative of the data from individual units, and examples of each response type are shown in Fig. 2. Specifically, examples of responses that appeared “phasic” are shown [e.g., on response alone (Fig. 2A), off response alone (Fig. 2B), and on and off response (Fig. 2C)]. Examples of units with more complex temporal response patterns were also seen (Fig. 2D).
Fig. 2.
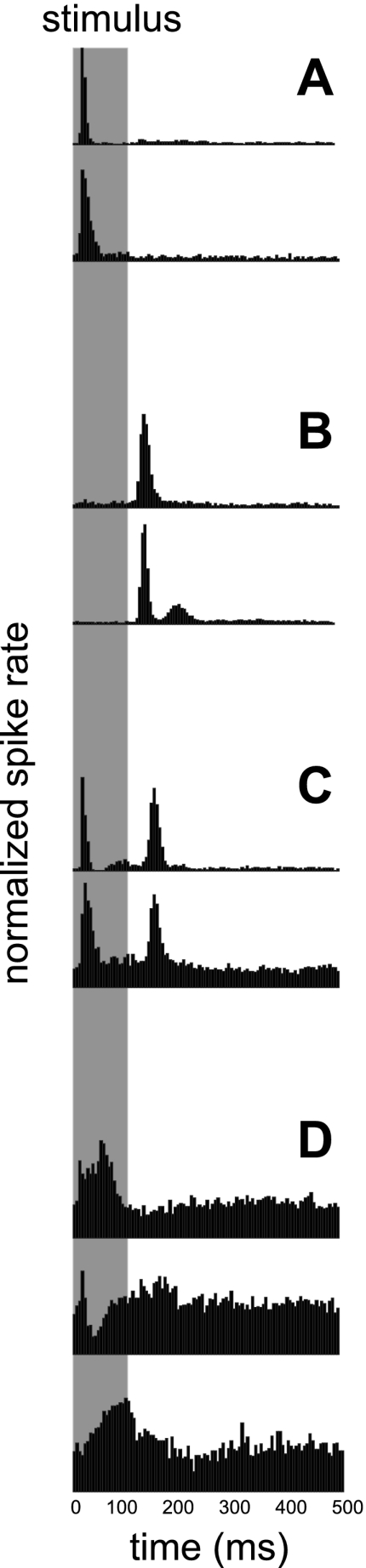
Examples of PSTHs for individual A1 units with a significant on response alone (A), a significant off response alone (B), a significant on and off response (C), and a complex temporal response pattern (D). The stimulus duration is represented by the light gray bar.
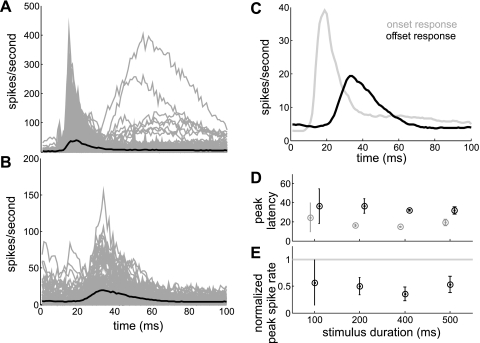
To compare the time course of on (Fig. 3A) and off (Fig. 3B) responses, mean responses to stimulus durations of ≥100 ms were superimposed by aligning the stimulus onset and offset at time 0 (Fig. 3C). The on response was characterized by a shorter rise time (average “peak latency” ∼19 ms after sound onset; Fig. 3C) than the off response (average peak latency ∼33 ms after sound offset; Fig. 3C), regardless of stimulus duration (Fig. 3D). Also independent of stimulus duration (Fig. 3E), the “peak spike rate” of the off response (19.6 spikes/s; Fig. 3C) was about half that of the on response (39.9 spikes/s; Fig. 3C). The on response decayed to half of the peak rate within 8 ms (“half decay time,” 27 ms after the sound onset; Fig. 3C). In contrast, the off response took twice as long (19 ms) to decay to half of the peak value (52 ms after the sound offset; Fig. 3C). The spike rate at the offset of the stimulus was similar to the spike rate 100 ms after the onset of the stimulus (4.9 and 5.0 spikes/s, respectively). These rates were higher than spike rates at the onset of the stimulus and 100 ms after the offset of the stimulus (Fig. 3C). Together these data suggest that, although the average cortical response to the stimulus appeared “phasic,” the spike rate did not fall to the background spike rate during the stimulus period. Indeed, clear examples of “sustained” and other temporally diverse response patterns were occasionally seen (Fig. 2D).
Fig. 3.
Spike rate for individual units (gray lines) and averaged across the population of units (black line) displaying either significant on responses (A) or off responses (B), plotted against time after the stimulus onset (A) and offset (B). C: mean spike rates across all units within onset (gray line)- and offset (black line)-response windows are superimposed by aligning the stimulus onset and offset at time 0 on the x-axis. D: mean (±SD) peak latency against stimulus duration for on (gray circles) and off (black circles) responses. E: mean (±SD) normalized peak spike rate against stimulus duration for off responses (black circles). The peak spike rate for the off response was normalized against the peak spike rate for the on response (gray line).
The principal focus of the present study was to characterize binaural sensitivity following the onset and offset of the stimulus. Binaural sensitivity varies between neurons, regardless of the response period, which complicates the comparison of sensitivity between on and off responses derived from different recording locations. To overcome this problem, we compared binaural sensitivity between on and off responses of the same unit. Therefore, for initial analysis, units were only included that had significant on and off responses to binaural stimulation (n = 154). These units represented only a subset of the total number of binaurally responsive neurons that were recorded, and it is not known to what extent they contribute to sound localization coding. In subsequent analysis, described in a separate section below, we therefore included units with 1) a significant on response alone (n = 322) and 2) a significant off response alone (n = 71).
Factors Affecting Binaural Sensitivity: On and Off Responses
Units with significant on and off responses.
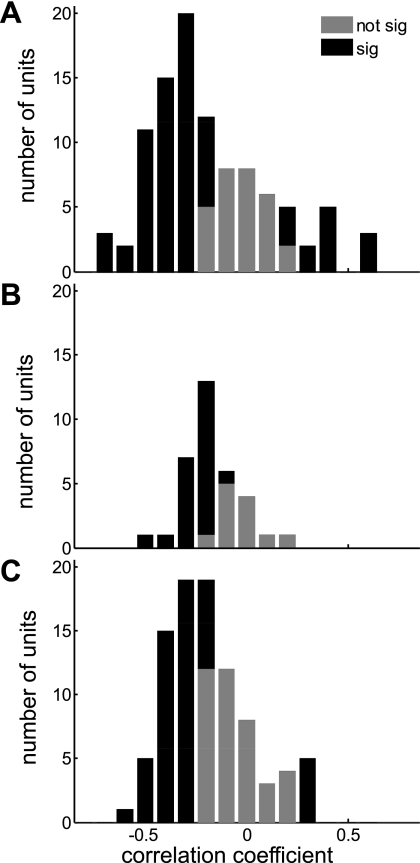
Binaural response functions to eITD, fITD, and ILD stimuli of ≥100 ms in duration were generated from mean spike rates averaged across the onset and offset recording windows (Fig. 4). Frequency tuning curves were generated for the same response periods. Binaural sensitivity (eITD, fITD, and/or ILD sensitivity) was assessed by determining whether a Poisson regression model up to 4th order fitted the data significantly better than a null model that assumed no effect of stimulus (see methods). Of the units that showed significant sensitivity to binaural stimulation, 66.4% were sensitive within the on response, 33.6% were sensitive within the off response, and 22% were sensitive in both. In the latter group, we compared binaural on and off responses, using a bootstrap test. We found that sensitivity to binaural stimuli changed significantly (P < 0.05) from the on response to the off response in 96.6% of cases. Binaural on responses were generally negatively correlated with off responses within individual units. This is illustrated in Fig. 5, which shows the distribution of correlation coefficients between trial-averaged on and off response matrices (such as those illustrated in Fig. 4, J, K, M, and N) in histogram form. Specifically, the correlation coefficients for the majority of units lay between 0 and −0.5 (Fig. 5). As Fig. 5A shows, 58% of neurons that exhibited onset and offset responses to our ILD stimuli had significantly anticorrelated onset and offset ILD tuning functions, compared with only 19.5% that showed significant positive correlation, while 22.5% exhibited no significant correlation. Similarly, of the units that exhibited on and off responses to our eITD stimuli, 65% had significantly anticorrelated on and off responses and 1% had significantly positively correlated on and off responses (Fig. 5B), while for the units that showed on and off responses to the fITD stimuli, 58% exhibited significant negative correlations between on and off responses, compared with only 12% that showed significant positive correlations (Fig. 5C). To ensure that this test was sensitive to the shape of the binaural response functions of the on and off responses, but not to differences in mean firing rate during the on and off periods, we normalized on and off response functions to a root mean square value of 1 and, subsequently, found that sensitivity to binaural stimuli changed significantly from the on response to the off response in 88.3% of cases (data not shown). What causes this predominant anticorrelation between onset and offset responses is at present unclear, but we discuss possible mechanisms below.
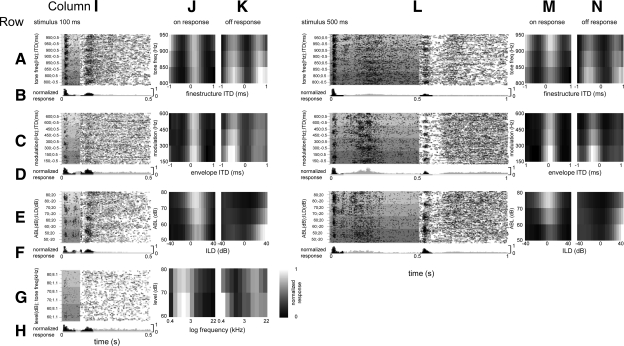
Fig. 4.
An example of a single unit's responses to fine-structure interaural time delay (fITD; rows A and B), envelope ITD (eITD; rows C and D), and interaural level difference (ILD; rows E and F) stimuli of 100-ms (columns I–K) and 500-ms (columns L–N) duration. For the same unit, frequency tuning in response to stimuli of 100-ms duration is also shown (rows G and H; columns I–K). Dot-raster plots show spike timing as black dots (rows A, C, E, and G; columns I and L), with time and stimulus plotted on the x- and y-axes, respectively. In the same plots, the background bars of different shades of gray represent stimulus duration and, along the y-axis, the different stimuli, arranged as follows: row A, columns I and L: from higher- to lower-frequency tones (lighter to darker gray shaded bars, respectively) and from positive to negative fITDs (upper to lower half of each shaded bar, respectively); row C, columns I and L: from higher to lower frequency of modulation (lighter to darker gray shaded bars, respectively) and from positive to negative eITDs (upper to lower half of each shaded bar, respectively); row E, columns I and L: from higher to lower average binaural levels (ABLs) (lighter to darker gray shaded bars, respectively) and from positive to negative ILDs (upper to lower half of each shaded bar, respectively) (note that ipsilateral intensity increases along a diagonal from bottom left to top right corners of the plot and contralateral intensity increases along a diagonal from bottom right to top left corners); and row G, column I: from higher to lower tone levels (lighter to darker gray shaded bars, respectively) and from higher to lower tone frequencies (upper to lower half of each shaded bar, respectively). PSTHs are shown below each dot-raster plot (rows B, D, F, and H; columns I and L), where black and gray bars correspond to PSTH bins that were used to generate the binaural response matrices (columns J, K, M, and N) and nonselected bins, respectively. Binaural response matrices are shown for on (columns J and M) and off (columns K and N) responses to stimuli of 100-ms (columns J and K) and 500-ms (columns M and N) duration, where black and white cells correspond to normalized responses of 0 and 1, respectively.
Fig. 5.
Analysis of units with on-off responses: distribution of correlation coefficients between on and off response sensitivity to ILDs (A), eITDs (B), and fITDs (C). Black bars represent units for which the tuning of on and off responses is significantly correlated (where correlations could be positive or negative). Gray bars represent units for which the correlation between on and off response tuning failed to reach statistical significance.
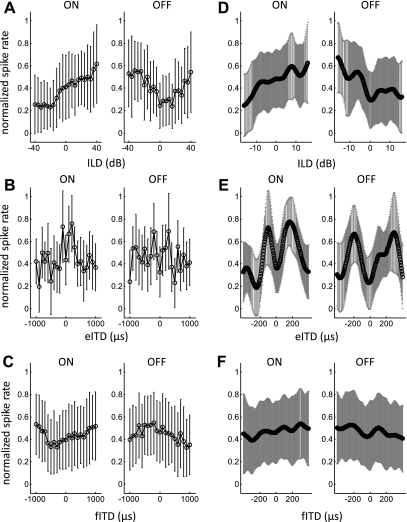
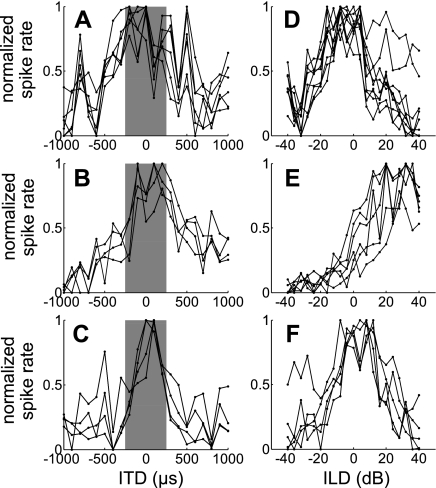
In Fig. 6 we examine trends across the whole sampled neural population by plotting mean, normalized firing rate (±SD) for all units that showed significant sensitivity to binaural stimulation during both the on and off response. This is shown for the full binaural stimulus range tested in Fig. 6, A–C, whereas in Fig. 6, D–F, responses were restricted to approximate the “physiological range” of ILDs and ITDs experienced by normal adult ferrets because of their head size (Hartley et al. 2010; Schnupp et al. 2003). Note that the ITDs generated by a sound stimulus tend to be largely independent of sound frequency, so that the physiological range can be defined in a manner that does not need to take account of stimulus frequency content or neural frequency sensitivity. However, ILDs are frequency dependent. The range of ILD values shown in Fig. 6D should therefore be understood to approximate the physiological range. For on responses, the mean firing rate increased across the physiological range of ILDs and fITDs, toward the side contralateral to the recording site, whereas the mean off response increased toward the ipsilateral side (Fig. 6, D and F). The population average eITD tuning curves exhibited peaks both ipsilaterally and contralaterally to the recording site (Fig. 6E), but peak eITD values for on responses were closer to the midline (−100 and 170 μs) than those seen for off responses (−195 and 295 μs).
Fig. 6.
Analysis of units with on-off responses: mean (±SD) normalized firing rate for on and off responses to ILD (A), eITD (B), and fITD (C) stimuli. Mean (±SD) response functions, interpolated within the physiological range of ILDs (D), eITDs (E), and fITDs (F), are also shown.
For all units with significant on and off responses (Fig. 1D), an index of ILD, eITD, and fITD sensitivity was calculated across all recording sweeps with the formula:
where rmax and rmin represent the maximum and minimum firing rate averaged across all repeat presentations of the same stimulus, respectively, and σmax and σmin represent the corresponding standard deviations.
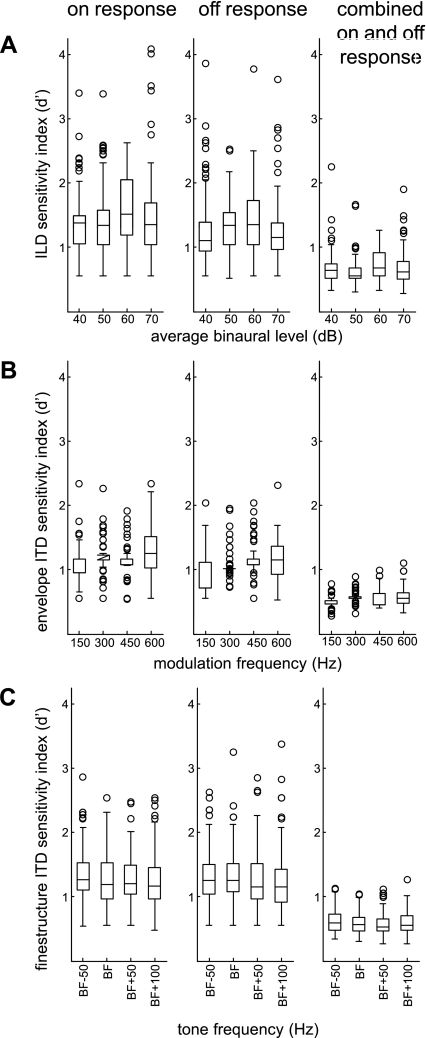
Figure 7 plots the ILD and ITD sensitivity index for on responses, off responses, and combined on and off responses of the units showing significant binaural sensitivity during on and off responses. Compared with on responses and off responses alone, the sensitivity index was lower for combined on and off responses. The latter result is consistent with differences in binaural sensitivity between on and off responses shown in Figs. 5 and 6.
Fig. 7.
Analysis of units with on-off responses: A: box plots of ILD sensitivity index plotted against ABL for on responses, off responses, and combined on and off responses of these units. B: box plots of eITD sensitivity index plotted against modulation frequency for on responses, off responses, and combined on and off responses. C: box plots of fITD sensitivity index plotted against tone frequency for on responses, off responses, and combined on and off responses.
Based on normalized spike rates the sensitivity index was higher for off responses compared with on responses (data not shown). On the other hand, based on raw spike counts, the mean sensitivity index was higher for on responses compared with off responses (Fig. 7). Specifically, the mean ILD sensitivity index was 1) higher for on responses compared with off responses and 2) higher in response to 60-dB average binaural level (ABL) compared with other ABLs (Fig. 7A). A repeated-measures analysis of variance (ANOVA) revealed a significant main effect for response period (on vs. off; F1,83 = 16.6, P <0.001) and ABL (F3,81 = 37.3, P <0.01) but no significant interaction between response period and ABL (F3,81 = 1.8, P = 0.1). Post hoc analyses, with Bonferroni correction for the number of comparisons made, revealed that ILD sensitivity was significantly greater in response to 60-dB ABL compared with 40-dB (mean difference 0.19; P < 0.01), 50-dB (mean difference 0.13; P < 0.05), and 70-dB (mean difference 0.17; P < 0.05) ABL stimuli.
Figure 7B plots the eITD sensitivity index for on and off responses. On average, the eITD sensitivity index was 1) higher for on responses compared with off responses and 2) lower in response to 150-Hz modulation compared with higher modulation rates. A repeated-measures ANOVA revealed a significant main effect for response period (F1,56 = 20.5, P < 0.001) and modulation frequency (F3,54 = 11.8, P < 0.001) and a significant interaction between response period and modulation frequency (F3,54 = 4.6, P < 0.01). Post hoc analyses, with Bonferroni correction for the number of comparisons made, revealed that eITD sensitivity was significantly lower in response to 150-Hz modulation compared with 300-Hz (mean difference 0.14; P < 0.001), 450-Hz (mean difference 0.15; P < 0.001), and 600-Hz (mean difference 0.23; P < 0.001) modulation.
Figure 7C plots the fITD sensitivity index for on and off responses. A repeated-measures ANOVA revealed no significant main effect of response period (F1,123 = 0.1, P = 0.8) or tone frequency (F3,121 = 1.9, P = 0.1).
Units with significant on response alone and significant off response alone.
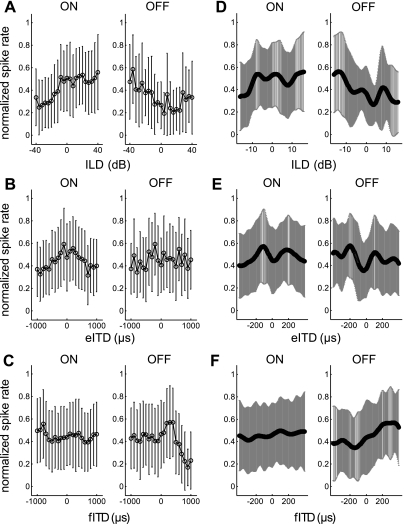
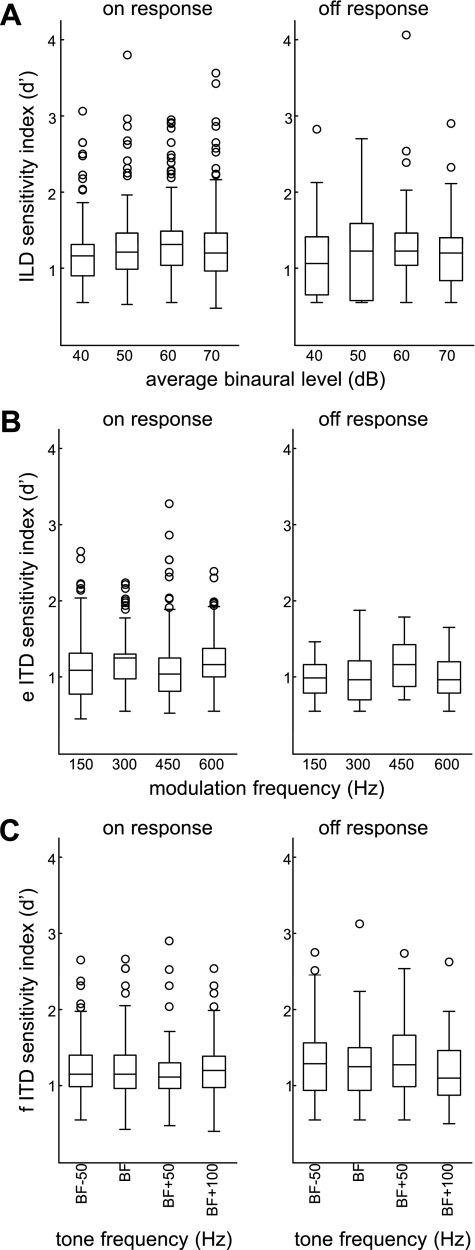
Figure 8 plots mean ILD and ITD responses for units with significant on responses alone (n = 322) and for the smaller number of units with significant off responses alone (n = 71). On responses increased across the physiological range of ILDs toward the side contralateral to the recording site, whereas off responses increased toward the ipsilateral side (Fig. 8D). Both ipsilateral and contralateral to the recording site, the mean firing rate peaked in response to eITDs (Fig. 8E). Interpolation analysis suggested that eITDs of approximately −110 and 180 μs would elicit peaks in the mean firing rate for on responses, whereas peak off responses would be elicited by eITDs of approximately −200 and 105 μs. Both on and off responses increased across the physiological range of fITDs, toward the side contralateral to the recording site (Fig. 8F). Figure 9 plots the ILD and ITD sensitivity index for units with significant on responses alone and units with off responses alone. Although the sensitivity index was similar between groups, this analysis was complicated by differences in recording location and sample size.
Fig. 8.
Analysis of units with on responses alone and units with off responses alone: mean (±SD) normalized firing rate to ILD (A), eITD (B), and fITD (C) stimuli. Mean (±SD) response functions, with interpolation within the physiological range of ILDs (D), eITDs (E), and fITDs (F), are also shown.
Fig. 9.
Left: binaural sensitivity of units with a significant on response alone: box plots of ILD sensitivity index plotted against ABL (A), eITD sensitivity index plotted against modulation frequency (B), and fITD sensitivity index plotted against tone frequency (C). Right: for units with a significant off response alone, box plots of ILD (A), eITD (B), and fITD (C) sensitivity index.
Factors Not Affecting Binaural Sensitivity: Cortical Depth and Surface Location
Single-shank probes with 16 recording sites were used in all experiments, and care was taken to insert the probe perpendicular to the cortical surface with the most proximal site positioned at the surface of the cortex. Subsequently, the depth of recording sites is related to the cortical layers (Dahmen et al. 2008). In most recordings, binaural tuning varied little with recording depth (Fig. 10). Although this study was not designed to study systematic variations of binaural sensitivity in different regions of the auditory cortex, topographic organization of binaural sensitivity across the surface of ferret A1 was not evident. Specifically, based on surface landmarks, recording locations were classified into caudal or rostral and dorsal and ventral. Subsequently, binaural interactions were compared between these locations. No topography was evident from this analysis.
Fig. 10.
Examples of ITD (A–C), and ILD (D–F) response functions derived from evenly spaced recording locations in 3 different electrode penetrations positioned orthogonal to the cortical surface. ITD and ILD functions within each row are derived from different recording locations along the same electrode penetration. With a 1,500-μm distance between the deepest and most superficial recording sites, it was possible to position the recording sites such that they spanned the cortical layers. The gray bar represents the physiological range of ITDs.
Distinct ITD Response Classes in A1?
As described in methods, we used cluster analyses of the binaural response functions in principal component space to investigate the diversity of binaural responses. A meaningful cluster analysis in a potentially high-dimensional space requires a large data set; hence only the eITD data set, comprising 128 units, was investigated in this manner.
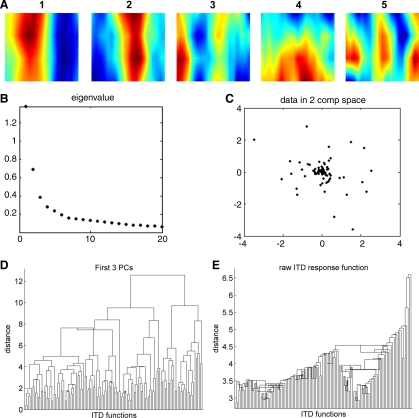
The first step of the cluster analysis was a dimensionality reduction using PCA. Each eITD function was measured at 76 stimulus parameters (19 ITD values by 4 modulation rates). However, the first 6 principal components (PCs) of these 76 dimensional eITD functions were sufficient to account for most (59.3%) of the variance in eITD functions across the population. The variance explained by PCs beyond the sixth then drops off slowly (see Fig. 11B), which probably reflects the fact that much of the variance in the observed response functions is due to variability in the neural discharges (“noise”) while the underlying structure of the eITD functions is largely captured by the first six PCs. Indeed, the eigenvectors of the first few PCs are interpretable in terms of simple physiological response features, as can be seen in Fig. 11A. PC 1 distinguishes neurons preferring ipsilateral stimuli from those preferring contralateral stimuli. PCs 2 and 3 discriminate “peak-type” from “trough-type” ITD tuning, while PC 4 captures preferences for low modulation frequencies. Finally, PC 5 can be thought of as a “higher harmonic” of PCs 1 and 2, capable of capturing steeper phasic ITD tuning curves that one might expect to see in neurons with a higher BF.
Fig. 11.
Principal component analysis of sensitivity to envelope ITDs. A: the eigenvectors of the first few principal components (PCs) are interpretable in terms of physiological tuning characteristics (see text). B: proportion of variance between eITD functions in our data set explained by the first 20 PCs. C: plotting the eITD functions in a space spanned by the first few PCs reveals no obvious clusters [illustrated here for the first 2 PCs (2 comp)]. D and E: dendrograms showing the result of a hierarchical clustering of the eITD functions either according to city-block distances in the space spanned by the first 3 PCs (D) or by Euclidean distances of the raw eITD tuning curves (E).
Note that the results in the cluster analysis shown in Fig. 11 are based on eITD functions derived from each neuron's full response (onset and offset combined), but a cluster analysis based on onset responses alone yielded essentially identical results (not shown).
The fact that the PCs appear to correspond to sensible “physiological features” suggests that they might form appropriate classification criteria, but plotting the ITD functions in the space spanned by the first few PCs revealed no obvious subdivision of the data into separate clusters (Fig. 11C), nor did a hierarchical cluster analysis of the data. Figure 11D shows in dendrogram form the results of a hierarchical clustering analysis of the ITD functions based on their Euclidean distances in the space spanned by the first three PCs. The clustering was carried out as described by Martinez and Martinez (2002), using an average linkage method. In Fig. 11D, the 128 ITD response functions of the data set are arranged along the x-axis by proximity of the eITD function in a space spanned by the first 3 PCs. The lengths of the vertical lines connecting the eITD functions indicate their distances in PC space, as shown on the y-axis. The hierarchical clustering algorithm groups eITDs by successive paring and grouping of the nearest neighbors. Note that the distances between the emerging groups are no longer than those between individual pairs of eITD functions, which indicates that the data cannot be separated into discrete clusters (i.e., the dissimilarity between clusters is no greater than the dissimilarity between neighboring eITD functions within a cluster). Clustering using city-block metrics instead of Euclidean distances or centroid linkage instead of average linkage similarly yielded no strong evidence for clustering. To illustrate this, a dendrogram obtained by hierarchical clustering according to Euclidean distances of the raw eITD functions is shown in Fig. 11E. Therefore, these analyses suggest that cortical ITD responses are best described as a continuum of response types, rather than discrete classes of ITD functions.
DISCUSSION
Binaural Sensitivity Within On and Off Responses
In sensory brain regions, including the visual, somatosensory, and auditory cortex, neurons can respond to the termination of a stimulus as well as its onset. Qin et al. (2007, 2008, 2009) have shown that sensitivity to sounds can vary between the on and the off responses of cat A1 neurons. A number of possible mechanisms have been proposed to account for on and off responses in the auditory cortex. Qin and colleagues suggested that on and off responses are driven by presynaptic neurons that respond both to the onset and to the offset of the stimulus, but it has also been suggested that off responses may represent release of inhibition or a rebound from sustained hyperpolarization (Calford and Webster 1981; Takahashi et al. 2004; Volkov and Galazjuk 1991). Rebound from inhibition could contribute to the predominantly anticorrelated on and off receptive fields that we have described here if we assume that weak or absent on responses were caused by strong inhibition that temporally overlaps with any onset or sustained part of the response. While our results are therefore compatible with the idea of rebound inhibition playing an important role, recent results of other researchers would argue against such a mechanism. In particular, Scholl and colleagues (2010) used in vivo whole cell recordings in rat A1 to measure the synaptic inhibition evoked by free-field stimulation. They found that this inhibition was typically transient, suggesting that a rebound recovery from inhibition would occur too early to account for off responses. Furthermore, their data showed that on and off responses had distinct excitatory-inhibitory balance and frequency tuning: off responses were typically tuned 1–2 octaves above on responses, and they showed that an on-on sequence causes complete forward suppression whereas an off-on sequence causes no suppression at all. Together this evidence suggests that on and off responses are driven by largely nonoverlapping sets of synapses.
Although a number of studies have examined on and off responses of cortical neurons to other sound attributes, there have been few attempts to investigate their sensitivity to binaural cues in different response periods. Consistent with previous studies, we found that binaural sensitivity within the off response was frequently different from, and indeed often negatively correlated with, that of the on response. In agreement with previous studies we also found that off responses 1) occurred over a longer duration and 2) had a smaller amplitude compared with on responses (Volkov and Galazjuk 1991). Our finding that off responses were present in ∼30% of units is also consistent with the values of 31% reported in ketamine-anesthetized cats (Volkov and Galazjuk 1991) and 28% in halothane-anesthetized cats (Moshitch et al. 2006). A higher prevalence of off responses of nearly 60% has been reported in awake cats (Qin et al. 2007). Although it has been suggested that the temporal diversity of cortical responses is greater in awake compared with anesthetized animals (Fishman and Steinschneider 2009; Recanzone 2000a; Scott et al. 2007; Wang et al. 2005), cortical neurons with complex temporal response patterns have been found in barbiturate (Mrsic-Flogel et al. 2005)- and ketamine (Bizley et al. 2005)-anesthetized ferrets. Furthermore, Walker and colleagues (2008) recorded cortical responses to natural sounds in ketamine-anesthetized and awake ferrets, and, apart from stronger on and off responses in the awake state, few differences were seen. Likewise, Dahmen and colleagues (2008) found similar stimulus timing-dependent plasticity in the auditory cortex of ketamine-anesthetized and awake ferrets. Conversely, neural sensitivity in cortex can alter significantly in the awake state, when an animal engages in a task (Fritz et al. 2005) and attends to a stimulus (Fritz et al. 2007). These findings suggest that, compared with the introduction of anesthesia, behavioral context has a greater influence on cortical firing. As yet, the temporal response properties of cortical neurons to binaural stimulation in awake animals have not been characterized in detail.
Distinct Classes of Binaural Responses in A1?
In agreement with early electrophysiological studies of binaural interactions in anesthetized cats, we found that neurons within a cortical column, running orthogonal to the cortical surface, have similar binaural response properties (Abeles and Goldstein 1970; Imig and Adrian 1977). Studies in anesthetized ferrets (Kelly and Judge 1994; Phillips et al. 1988), albino rats (Kelly and Sally 1988), guinea pigs (Rutkowski et al. 2000), cats (Nakamoto et al. 2004; Reale and Kettner 1986), and owl monkeys (Recanzone et al. 1999), which sampled different sites across the surface of the cortex, suggested that binaural responses may be organized within tightly arranged “clusters” or “patches” of response types. However, this clustering of response types is likely to reflect the way in which the response types were subsequently classified (Campbell et al. 2006). Indeed, recent studies have adopted progressively more complex classification systems, involving as many as 14 classes of binaural response types (Rutkowski et al. 2000; Zhang et al. 2004). The use of such complex classification systems is associated with finding evidence for well-localized patches of binaural response types (Nakamoto et al. 2004; Reale and Kettner 1986; Rutkowski et al. 2000), whereas less complex classification schemes tend to produce larger patches of binaural response types across the cortical surface, arguably because they tend to include more disparate response types within a single group.
PCA can be useful in the search for “natural” subdivisions or clusters within large data sets. PCA identifies directions of maximal variance within the data set, and since systematic differences between clusters can be expected to contribute toward much of the total variance in the data set, these directions of maximal variance often correspond to distinguishing features of the data sets underlying classes. Campbell and colleagues (Campbell et al. 2006) used this analysis in a recent study of cortical ILD sensitivity in ferret A1. Although their analysis indicated that a small number of PCs could capture most of the diversity of observed binaural response properties of the neurons, it nevertheless did not reveal any natural subdivisions of the population of observed binaural response properties. They concluded that responses to ILDs were probably best characterized by a continuum of response types, rather than discrete classes. Similarly, other studies have proposed that discrete ILD response types within A1 are unlikely (Irvine et al. 1996; Semple and Kitzes 1993).
Of the few studies that have investigated ITD sensitivity in A1 (Coffey et al. 2006; Fitzpatrick and Kuwada 2001; Kelly and Phillips 1991; Reale and Brugge 1990), most have relied upon classification schemes that have been developed through recordings from auditory brain stem nuclei. Whereas there is some evidence to suggest that binaural response classes within the auditory brain stem and midbrain may correspond with anatomically separable groups of cells (Chase and Young 2005; Fuzessery and Hall 1999; Loftus et al. 2010; McAlpine et al. 1996; Moore and Irvine 1981; Semple and Aitkin 1979; Yin et al. 1985), this seems less likely to be the case within A1. Indeed, the cluster analyses carried out in the present study, both on the raw response functions and on the representations of the data in a lower-dimensional principal component space, revealed no evidence for the existence of discrete ITD response classes. Consistent with previous reports of a continuum of ILD responses within A1 (Campbell et al. 2006), we found a similar continuous distribution of ITD response types. Indeed, a continuum of binaural interactions in A1 is consistent with the range of spatial receptive fields reported in both cats (Imig et al. 1990; Rajan et al. 1990; Stecker et al. 2005) and ferrets (Mrsic-Flogel et al. 2005).
Our data also suggest that the dynamic range of ITDs and ILDs within A1 extends beyond the physiological range of binaural cue values that the animal is likely to experience in its natural environment. These binaural functions vary along a continuum from those preferentially responding to ipsilateral stimulation to the majority that prefer contralateral sound locations. Functionally, this is consistent with the idea that auditory space may be encoded by overlapping populations of neurons that predominantly convey spatial information via the slope, rather than the peak, of the response functions (Harper and McAlpine 2004; McAlpine et al. 2001).
Functional Significance of Cortical On and Off Responses
Psychophysical experiments show that sound offset serves as an important acoustic cue in the acoustic startle reflex (Ison and Allen 2003), perception of sound duration (Schlauch et al. 2001), consonant identification (Pind 1998), and perceptual grouping, auditory scene analysis, and auditory lateralization (Darwin and Carlyon 1995). It has been suggested that the off response to a stimulus may be the neural correlate of a perceptual “temporal edge” that serves to segregate sounds into distinct groups (Bregman et al. 1994; Plack and White 2000). Indeed, if a noise is used to mask temporal gaps between a series of tones, a continuity illusion can arise in which the listener appears to hear a continuous tone (Tougas and Bregman 1990), and a neural substrate for this has been described in A1 (Petkov et al. 2007).
In terms of the binaural cues used in the present study, the contribution of onset and offset cues to auditory spatial perception has been extensively investigated (Moore 1997). In one such study, Stecker and Hafter (2009) showed that listeners' ability to localize a train of clicks is characterized by weighting of 1) the onset click and 2) late-arriving sounds within the click train. Spatial offset cues may also contribute to auditory memory since it is known that more recent events are recalled more accurately than preceding ones (Glanzer and Cunitz 1966). Furthermore, on and off responses to binaural stimulation may contribute to motion detection (Stecker and Hafter 2009). Considering the different binaural tuning that we observed for on and off responses, and the contribution of the onset and offset location of a stimulus to the perceived location of a sound (Stecker and Hafter 2009), it is possible that motion may be perceived when the on and off responses to a stimulus differ in location. Indeed, onset and offset cues may even contribute to nonauditory processes (i.e., cognitive or motor) by biasing a response to a sound, as opposed to contributing to sensory perception itself. For example, it is possible that motor responses, such as head turns or arm movements, could be fine-tuned by temporal components of the response to an auditory spatial stimulus.
GRANTS
This work was supported by the Wellcome Trust through a Clinician Scientist Fellowship (D. E. H. Hartley), a 4-year Studentship (J. C. Dahmen), and a Principal Research Fellowship (A. J. King).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Abeles M, Goldstein MH., Jr Functional architecture in cat primary auditory cortex: columnar organization and organization according to depth. J Neurophysiol 33: 172–187, 1970 [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. Enhancing sensitivity to interaural delays at high frequencies by using “transposed stimuli”. J Acoust Soc Am 112: 1026–1036, 2002 [DOI] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Nelken I, King AJ. Functional organization of ferret auditory cortex. Cereb Cortex 15: 1637–1653, 2005 [DOI] [PubMed] [Google Scholar]
- Bregman AS, Ahad PA, Kim J. Resetting the pitch-analysis system. 2. Role of sudden onsets and offsets in the perception of individual components in a cluster of overlapping tones. J Acoust Soc Am 96: 2694–2703, 1994 [DOI] [PubMed] [Google Scholar]
- Brugge JF, Reale RA, Hind JE, Chan JC, Musicant AD, Poon PW. Simulation of free-field sound sources and its application to studies of cortical mechanisms of sound localization in the cat. Hear Res 73: 67–84, 1994 [DOI] [PubMed] [Google Scholar]
- Calford MB, Webster WR. Auditory representation within principal division of cat medial geniculate body: an electrophysiology study. J Neurophysiol 45: 1013–1028, 1981 [DOI] [PubMed] [Google Scholar]
- Campbell RA, Schnupp JW, Shial A, King AJ. Binaural-level functions in ferret auditory cortex: evidence for a continuous distribution of response properties. J Neurophysiol 95: 3742–3755, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RA, Schulz AL, King AJ, Schnupp JW. Brief sounds evoke prolonged responses in anesthetized ferret auditory cortex. J Neurophysiol 103: 2783–2793, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase SM, Young ED. Limited segregation of different types of sound localization information among classes of units in the inferior colliculus. J Neurosci 25: 7575–7585, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey CS, Ebert CS, Jr, Marshall AF, Skaggs JD, Falk SE, Crocker WD, Pearson JM, Fitzpatrick DC. Detection of interaural correlation by neurons in the superior olivary complex, inferior colliculus and auditory cortex of the unanesthetized rabbit. Hear Res 221: 1–16, 2006 [DOI] [PubMed] [Google Scholar]
- Dahmen JC, Hartley DE, King AJ. Stimulus-timing-dependent plasticity of cortical frequency representation. J Neurosci 28: 13629–13639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin CJ, Carlyon RP. Auditory grouping. In: Hearing, edited by Moore BCJ. San Diego, CA: Academic, 1995, p. 387–420 [Google Scholar]
- Fishman YI, Steinschneider M. Temporally dynamic frequency tuning of population responses in monkey primary auditory cortex. Hear Res 254: 64–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick DC, Kuwada S. Tuning to interaural time differences across frequency. J Neurosci 21: 4844–4851, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, Shamma SA. Adaptive changes in cortical receptive fields induced by attention to complex sounds. J Neurophysiol 98: 2337–2346, 2007 [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, Shamma SA. Differential dynamic plasticity of A1 receptive fields during multiple spectral tasks. J Neurosci 25: 7623–7635, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzessery ZM, Hall JC. Sound duration selectivity in the pallid bat inferior colliculus. Hear Res 137: 137–154, 1999 [DOI] [PubMed] [Google Scholar]
- Glanzer M, Cunitz AR. Two storage mechanisms in free recall. J Verbal Learn Verbal Behav 5: 351–360, 1966 [Google Scholar]
- Griffin SJ, Bernstein LR, Ingham NJ, McAlpine D. Neural sensitivity to interaural envelope delays in the inferior colliculus of the guinea pig. J Neurophysiol 93: 3463–3478, 2005 [DOI] [PubMed] [Google Scholar]
- Harper NS, McAlpine D. Optimal neural population coding of an auditory spatial cue. Nature 430: 682–686, 2004 [DOI] [PubMed] [Google Scholar]
- Hartley DE, Vongpaisal T, Xu J, Shepherd RK, King AJ, Isaiah A. Bilateral cochlear implantation in the ferret: a novel animal model for behavioral studies. J Neurosci Methods 190: 214–228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques. J Neurophysiol 64: 915–31, 1990 [DOI] [PubMed] [Google Scholar]
- Hine JE, Martin RL, Moore DR. Free-field binaural unmasking in ferrets. Behav Neurosci 108: 196–205, 1994 [DOI] [PubMed] [Google Scholar]
- Imig TJ, Adrian HO. Binaural columns in the primary field (A1) of cat auditory cortex. Brain Res 138: 241–257, 1977 [DOI] [PubMed] [Google Scholar]
- Imig TJ, Irons WA, Samson FR. Single-unit selectivity to azimuthal direction and sound pressure level of noise bursts in cat high-frequency primary auditory cortex. J Neurophysiol 63: 1448–1466, 1990 [DOI] [PubMed] [Google Scholar]
- Irvine DR, Rajan R, Aitkin LM. Sensitivity to interaural intensity differences of neurons in primary auditory cortex of the cat. I. Types of sensitivity and effects of variations in sound pressure level. J Neurophysiol 75: 75–96, 1996 [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen P. A diminished rate of “physiological decay” at noise offset contributes to age-related changes in temporal acuity in the CBA mouse model of presbycusis. J Acoust Soc Am 114: 522–528, 2003 [DOI] [PubMed] [Google Scholar]
- Jackson L, Heffner H, Heffner R. Species differences in the upper limit of binaural phase discrimination (Abstract). Assoc Res Otolaryngol Abstr 19: 63, 1996 [Google Scholar]
- Jenkins WM, Merzenich MM. Role of cat primary auditory cortex for sound-localization behavior. J Neurophysiol 52: 819–47, 1984 [DOI] [PubMed] [Google Scholar]
- Joris P, Yin TC. A matter of time: internal delays in binaural processing. Trends Neurosci 30: 70–78, 2007 [DOI] [PubMed] [Google Scholar]
- Kavanagh GL, Kelly JB. Contribution of auditory cortex to sound localization by the ferret (Mustela putorius). J Neurophysiol 57: 1746–1766, 1987 [DOI] [PubMed] [Google Scholar]
- Kelly JB, Judge PW. Binaural organization of primary auditory cortex in the ferret (Mustela putorius). J Neurophysiol 71: 904–913, 1994 [DOI] [PubMed] [Google Scholar]
- Kelly JB, Phillips DP. Coding of interaural time differences of transients in auditory cortex of Rattus norvegicus: implications for the evolution of mammalian sound localization. Hear Res 55: 39–44, 1991 [DOI] [PubMed] [Google Scholar]
- Kelly JB, Sally SL. Organization of auditory cortex in the albino rat: binaural response properties. J Neurophysiol 59: 1756–1769, 1988 [DOI] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Oliver DL. Differential patterns of inputs create functional zones in central nucleus of inferior colliculus. J Neurosci 30: 13396–13408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez WL, Martinez AR. Computational Statistics Handbook with MATLAB. New York: Chapman & Hall/CRC, 2002 [Google Scholar]
- McAlpine D, Jiang D, Palmer AR. Interaural delay sensitivity and the classification of low best-frequency binaural responses in the inferior colliculus of the guinea pig. Hear Res 97: 136–152, 1996 [PubMed] [Google Scholar]
- McAlpine D, Jiang D, Palmer AR. A neural code for low-frequency sound localization in mammals. Nat Neurosci 4: 396–401, 2001 [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Clock AE, Xu L, Green DM. A panoramic code for sound location by cortical neurons. Science 264: 842–844, 1994 [DOI] [PubMed] [Google Scholar]
- Moore BCJ. Space perception. In: An Introduction to the Psychology of Hearing, edited by Moore BCJ. San Diego, CA: Academic, 1997, p. 213–244 [Google Scholar]
- Moore DR, Irvine DR. Development of responses to acoustic interaural intensity differences in the cat inferior colliculus. Exp Brain Res 41: 301–309, 1981 [DOI] [PubMed] [Google Scholar]
- Moshitch D, Las L, Ulanovsky N, Bar-Yosef O, Nelken I. Responses of neurons in primary auditory cortex (A1) to pure tones in the halothane-anesthetized cat. J Neurophysiol 95: 3756–3769, 2006 [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, King AJ, Jenison RL, Schnupp JW. Listening through different ears alters spatial response fields in ferret primary auditory cortex. J Neurophysiol 86: 1043–1046, 2001 [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, King AJ, Schnupp JW. Encoding of virtual acoustic space stimuli by neurons in ferret primary auditory cortex. J Neurophysiol 93: 3489–3503, 2005 [DOI] [PubMed] [Google Scholar]
- Nakamoto KT, Zhang J, Kitzes LM. Response patterns along an isofrequency contour in cat primary auditory cortex (AI) to stimuli varying in average and interaural levels. J Neurophysiol 91: 118–135, 2004 [DOI] [PubMed] [Google Scholar]
- Nodal FR, Kacelnik O, Bajo VM, Bizley JK, Moore DR, King AJ. Lesions of the auditory cortex impair azimuthal sound localization and its recalibration in ferrets. J Neurophysiol 103: 1209–1225, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodal FR, Keating P, King AJ. Chronic detachable headphones for acoustic stimulation in freely moving animals. J Neurosci Methods 189: 44–50, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CH, Lanyon RG, Schnupp JW, King AJ. Effects of altering spectral cues in infancy on horizontal and vertical sound localization by adult ferrets. J Neurophysiol 82: 2294–2309, 1999 [DOI] [PubMed] [Google Scholar]
- Petkov CI, O'Connor KN, Sutter ML. Encoding of illusory continuity in primary auditory cortex. Neuron 54: 153–165, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DP, Hall SE. Response timing constraints on the cortical representation of sound time structure. J Acoust Soc Am 88: 1403–1411, 1990 [DOI] [PubMed] [Google Scholar]
- Phillips DP, Judge PW, Kelly JB. Primary auditory cortex in the ferret (Mustela putorius): neural response properties and topographic organization. Brain Res 443: 281–294, 1988 [DOI] [PubMed] [Google Scholar]
- Pind J. Auditory and linguistic factors in the perception of voice offset time as a cue for preaspiration. J Acoust Soc Am 103: 2117–2127, 1998 [DOI] [PubMed] [Google Scholar]
- Plack CJ, White LJ. Perceived continuity and pitch perception. J Acoust Soc Am 108: 1162–1169, 2000 [DOI] [PubMed] [Google Scholar]
- Qin L, Chimoto S, Sakai M, Wang J, Sato Y. Comparison between offset and onset responses of primary auditory cortex ON-OFF neurons in awake cats. J Neurophysiol 97: 3421–3431, 2007 [DOI] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang J, Li S, Sato Y. Neural and behavioral discrimination of sound duration by cats. J Neurosci 29: 15650–15659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wang JY, Sato Y. Representations of cat meows and human vowels in the primary auditory cortex of awake cats. J Neurophysiol 99: 2305–2319, 2008 [DOI] [PubMed] [Google Scholar]
- Rajan R, Aitkin LM, Irvine DR, McKay J. Azimuthal sensitivity of neurons in primary auditory cortex of cats. I. Types of sensitivity and the effects of variations in stimulus parameters. J Neurophysiol 64: 872–887, 1990 [DOI] [PubMed] [Google Scholar]
- Reale RA, Brugge JF. Auditory cortical neurons are sensitive to static and continuously changing interaural phase cues. J Neurophysiol 64: 1247–1260, 1990 [DOI] [PubMed] [Google Scholar]
- Reale RA, Kettner RE. Topography of binaural organization in primary auditory cortex of the cat: effects of changing interaural intensity. J Neurophysiol 56: 663–682, 1986 [DOI] [PubMed] [Google Scholar]
- Recanzone GH. Response profiles of auditory cortical neurons to tones and noise in behaving macaque monkeys. Hear Res 150: 104–118, 2000a [DOI] [PubMed] [Google Scholar]
- Recanzone GH. Spatial processing in the auditory cortex of the macaque monkey. Proc Natl Acad Sci USA 97: 11829–11835, 2000b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Sutter ML, Beitel RE, Merzenich MM. Functional organization of spectral receptive fields in the primary auditory cortex of the owl monkey. J Comp Neurol 415: 460–481, 1999 [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Wallace MN, Shackleton TM, Palmer AR. Organisation of binaural interactions in the primary and dorsocaudal fields of the guinea pig auditory cortex. Hear Res 145: 177–189, 2000 [DOI] [PubMed] [Google Scholar]
- Schlauch RS, Ries DT, DiGiovanni JJ. Duration discrimination and subjective duration for ramped and damped sounds. J Acoust Soc Am 109: 2880–2887, 2001 [DOI] [PubMed] [Google Scholar]
- Schnupp JW, Booth J, King AJ. Modeling individual differences in ferret external ear transfer functions. J Acoust Soc Am 113: 2021–2030, 2003 [DOI] [PubMed] [Google Scholar]
- Scholl B, Gao X, Wehr M. Nonoverlapping sets of synapses drive on responses and off responses in auditory cortex. Neuron 65: 412–421, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BH, Malone BJ, Semple MN. Effect of behavioral context on representation of a spatial cue in core auditory cortex of awake macaques. J Neurosci 27: 6489–6499, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple MN, Aitkin LM. Representation of sound frequency and laterality by units in central nucleus of cat inferior colliculus. J Neurophysiol 42: 1626–1639, 1979 [DOI] [PubMed] [Google Scholar]
- Semple MN, Kitzes LM. Focal selectivity for binaural sound pressure level in cat primary auditory cortex: two-way intensity network tuning. J Neurophysiol 69: 462–473, 1993 [DOI] [PubMed] [Google Scholar]
- Stecker GC, Hafter ER. A recency effect in sound localization? J Acoust Soc Am 125: 3914–3924, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecker GC, Harrington IA, Middlebrooks JC. Location coding by opponent neural populations in the auditory cortex. PLoS Biol 3: e78, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Nakao M, Kaga K. Cortical mapping of auditory-evoked offset responses in rats. Neuroreport 15: 1565–1569, 2004 [DOI] [PubMed] [Google Scholar]
- Tougas Y, Bregman AS. Auditory streaming and the continuity illusion. Percept Psychophys 47: 121–126, 1990 [DOI] [PubMed] [Google Scholar]
- Volkov IO, Galazjuk AV. Formation of spike response to sound tones in cat auditory cortex neurons: interaction of excitatory and inhibitory effects. Neuroscience 43: 307–321, 1991 [DOI] [PubMed] [Google Scholar]
- Walker KM, Ahmed B, Schnupp JW. Linking cortical spike pattern codes to auditory perception. J Cogn Neurosci 20: 135–152, 2008 [DOI] [PubMed] [Google Scholar]
- Wang X, Lu T, Snider RK, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature 435: 341–346, 2005 [DOI] [PubMed] [Google Scholar]
- Yin TC, Hirsch JA, Chan JC. Responses of neurons in the cat's superior colliculus to acoustic stimuli. II. A model of interaural intensity sensitivity. J Neurophysiol 53: 746–758, 1985 [DOI] [PubMed] [Google Scholar]
- Zhang J, Nakamoto KT, Kitzes LM. Binaural interaction revisited in the cat primary auditory cortex. J Neurophysiol 91: 101–117, 2004 [DOI] [PubMed] [Google Scholar]
- Zwislocki J, Feldman R. Just noticeable differences in dichotic phase. J Acoust Soc Am 28: 860–864, 1956 [Google Scholar]