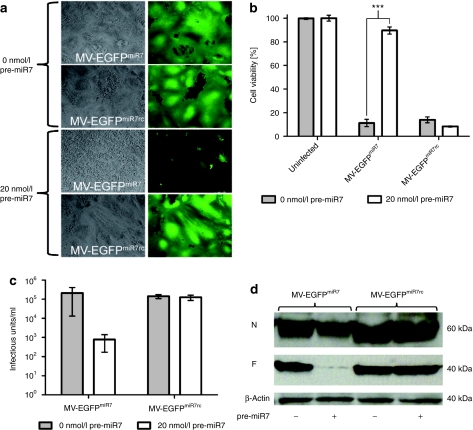
Figure 4.
miR7 regulates spreading, cytotoxicity, protein expression, and progeny virus production of MV-EGFPmiR7. (a) Vero cells were seeded in 6-well plates at a density of 6 × 105 cells/well. After attachment, cells were transfected with 0 or 20 nmol/l miR7 precursors and subsequently infected with MV-EGFPmiR7 or MV-EGFPmiR7rc at a multiplicity of infection (MOI) of 0.03. Forty hours postinfection, fluorescence microscopy was performed in order to detect progeny virus production and spread throughout the cell layer (×100 magnification). (b) Vero cells were seeded in 24-well plates at a density of 4 × 104 cells/well. Cells were transfected with 0 or 20 nmol/l of miR7 precursor molecules and infected subsequently with MV-EGFPmiR7 or MV-EGFPmiR7rc at an MOI of 0.03 followed by the XTT cell viability assay 87 hours postinfection. Statistical significance was tested using the two-tailed t-test. P values of <0.0001 are indicated with three asterisks. (c) Vero cells were seeded in 12-well plates at a density of 1.5 × 105 cells/well. After attachment, cells were transfected with either 0 or 20 nmol/l miR7 precursor molecules. Transfected cells were infected with MV-EGFPmiR7 or MV-EGFPmiR7rc at an MOI of 0.03. Forty-eight hours postinfection, the cells were scraped in their medium, subjected to one freeze-thaw cycle and progeny virus titers were determined. (d) Immunoblot of protein lysates derived from Vero cells infected with MV-EGFPmiR7 or MV-EGFPmiR7rc at an MOI of 0.03 in either the absence or presence of 20 nmol/l miR7 precursor molecules. Twenty-six hours postinfection cells were lysed, total protein was isolated and immunoblotted for β-actin, MV-N, and MV-F.