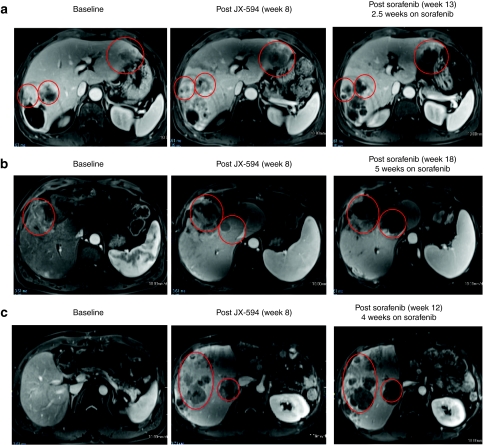
Figure 3.
JX-594 treatment of patients with advanced hepatocellular carcinoma cell may sensitize to subsequent sorafenib therapy. (a) Patient 1705 was treated with JX-594 at a dose level of 108 plaque-forming units (pfu) intratumorally for three treatments every 2 weeks (week 0, week 2, week 4). Sorafenib therapy was initiated at week 10.5. Antitumor response was evaluated by dynamic contrast-enhanced magnetic resonance imaging (MRI) at baseline, after treatment with JX-594 alone and after sorafenib initiation. Red circles indicate target tumors. The darker areas within the target tumors at week 13 represent significant increasing necrosis within the target tumors is seen at week 11, manifested by nonenhancement within these tumors. (b) Patient 1702 was treated with JX-594 at a dose level of 109 pfu intratumorally for three treatments every 2 weeks (week 0, week 2, week 4). Sorafenib therapy was initiated at week 13. Response was evaluated by dynamic MRI imaging at baseline, after treatment with JX-594 alone and after sorafenib initiation. Red circles indicate target tumors. Mild amount of necrosis is seen in the larger tumor at baseline. The necrosis increases somewhat following JX-594 alone, but significant necrosis is identified following sorafenib therapy. (c) Patient 1712 was treated with JX-594 at a dose level of 109 pfu intratumorally for three treatments every 2 weeks (week 0, week 2, week 4). Sorafenib therapy was initiated at Week 8. Response was evaluated by dynamic MRI imaging at baseline, after treatment with JX-594 alone and after sorafenib initiation. Red ovals indicate areas of the liver not injected with JX-594. These images demonstrate antitumor activity in noninjected lesions similar to that seen in injected lesions from (a) and (b). Progressively developing necrosis within these tumors is seen following JX-594 therapy alone and then sorafenib.