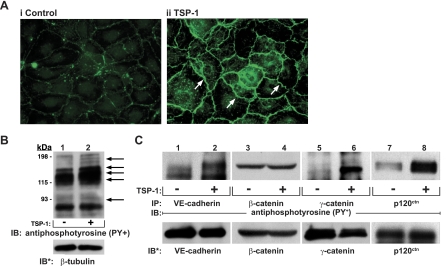
Fig. 2.
Identification of substrates for TSP1-induced protein tyrosine phosphorylation. A: postconfluent HMVEC-L monolayers were exposed for 1 h to TSP1 (30 μg/ml, i.e., 214 nM) (ii) or medium alone (i), after which they were probed with FITC-conjugated antiphosphotyrosine antibodies and processed for fluorescence microscopy. Arrows indicate increased phosphotyrosine signal at intercellular boundaries. Magnification = ×400. In other experiments, HMVEC-Ls were incubated for 1 h with TSP1 (30 μg/ml, i.e., 214 nM) (+) or medium alone (-) in the presence of vanadate (200 μM) and phenylarsine oxide (PAO) (1.0 μM) only during the last 0.25 h of incubation. B: TSP1-exposed and medium control endothelial cells (ECs) were lysed, and total cell lysates were resolved by SDS-PAGE, transferred to PVDF, and the blots probed with antiphosphotyrosine antibody. To indicate protein loading and transfer, the blots were stripped and reprobed with anti-β-tubulin antibody. C: lysates of TSP1-exposed and medium control ECs were immunoprecipitated with antibodies raised against vascular endothelial (VE)-cadherin, β-catenin, γ-catenin, or p120ctn. The immunoprecipitates were resolved by SDS-PAGE and transferred to PVDF, and the blots were probed with antiphosphotyrosine antibody. For normalization of phosphotyrosine signal to loading of the immunoprecipitated protein, blots were stripped and reprobed with the immunoprecipitating antibodies. IP, immunoprecipitate; IB, immunoblots; IB*, immunoblots after stripping. Each blot is representative of 3 independent experiments.