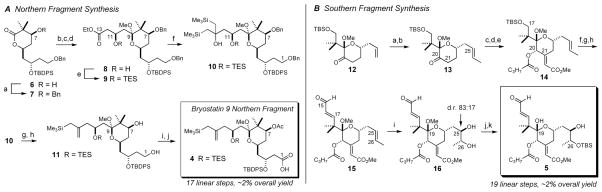
Scheme 1.
Synthesis of the bryostatin 9 northern and southern fragment coupling partnersa
a A Reagents and Conditions: (a) BnBr, NaHMDS, 5:1 THF:DMF, 0 °C, 90%; (b) ethyl acetoacetate (2.5 eq.), LDA (5.0 eq.), THF, −78 °C; (c) PPTS, MeOH, 40 °C, 84% over 2 steps; (d) NaBH4, EtOH, −15 °C, dr: 78:22, 61% isolated 8; (e) TESCl, imidazole, CH2Cl2, rt, 97%; (f) CeCl3·2LiCl, TMSCH2MgCl, THF, rt, 65%; (g) NaHMDS, THF, 0 °C, 91%; (h) lithium naphthalenide, THF, −30 °C → −10 °C, 87%; (i) TEMPO (30 mol %), PhI(OAc)2 (3 eq.), 4:1 MeCN:H2O, then NaH2PO4, NaClO2, 2-methyl-2-butene, 0 °C; (j) Ac2O, DMAP, CH2Cl2, 0 °C, then aq. NaHCO3, 57% over 2 steps. B Reagents and Conditions: (a) O3, CH2Cl2, −78 °C, then PPh3, rt, 98%; (b) I2CHCH3, CrCl2, DMF, THF, 0 °C, 76%, 93:7 E:Z; (c) K2CO3, methyl glyoxylate, THF:MeOH, rt, 81%; (d) NaBH4, CeCl3·7H2O, MeOH, −49 °C; (e) butyric anhydride, DMAP, CH2Cl2, rt, 91% over 2 steps; (f) 3HF·Et3N, THF, rt; (g) Dess-Martin periodinane, CH2Cl2, rt; (h) Z-1-bromo-2-ethoxyethylene, t-BuLi, Me2Zn, Et2O, −78 °C, then H3O+, 64% over 3 steps; (i) K2OsO4·2H2O (~0.5 mol %), DHQD2PYR (1.5 mol %), K2CO3, K3Fe(CN)6, 4 °C, 78%, 83:17 (R,R):(S,S); (j) p-TsOH, 4:1 MeCN:H2O, rt; (k) TBSCl, imidazole, CH2Cl2, 64% over 2 steps as a single diastereomer.