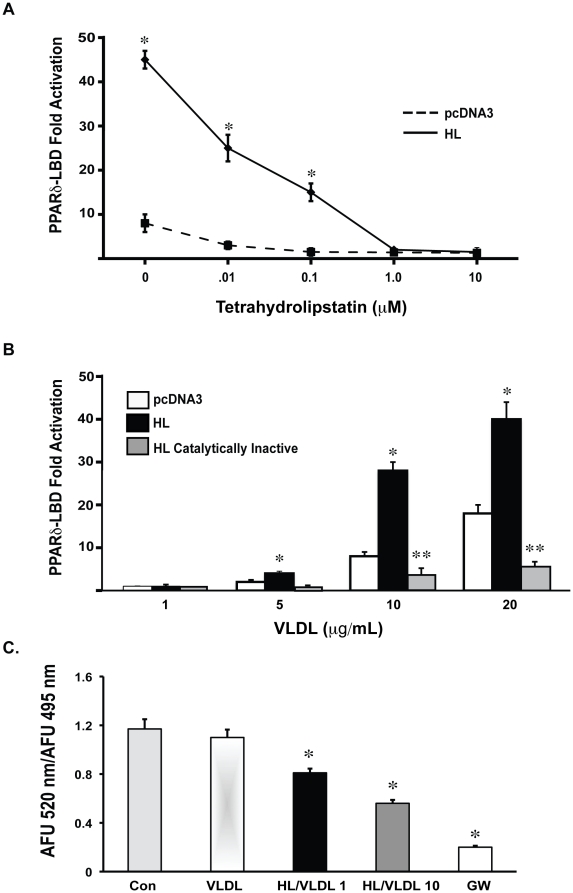
Figure 2. HL/VLDL activation of PPARδ depends on catalytic function.
(A) HEK-293 cells were transfected with PPARδ-LBD and pretreated with indicated concentrations of tetrahydrolipstatin (THL) or DMSO control for 30 minutes prior to stimulation with VLDL 30 µg/mL for 12 hours. Results expressed as mean fold change. *p<.05 vs. empty vector. (B) COS-7 cells were transfected with pcDNA, HL or catalytically inactive HL and stimulated with indicated concentrations of VLDL for 12 hours. *p<.05 vs. pcDNA. **p<.05 versus HL and pcDNA. (C) Time-resolved FRET PPARδ competitive binding assay: Recombinant HL was incubated with increasing concentrations of VLDL (1 µg/mL and 10 µg/mL) for 1 hour with recombinant GST-PPARδ-LBD, terbium-labeled anti-GST antibody and a fluorescein-labeled PPARδ synthetic agonist. FRET activity, measured by the emission ratio of fluorescence at 520 nm/495 nm, was inhibited in a VLDL concentration dependent manner in the presence of HL. The synthetic PPARδ ligand, GW501516, maximally inhibited FRET at 1 µM. *p<.05 compared to VLDL alone.