Figure 2. Characterization of protein-nanoparticle complexation.
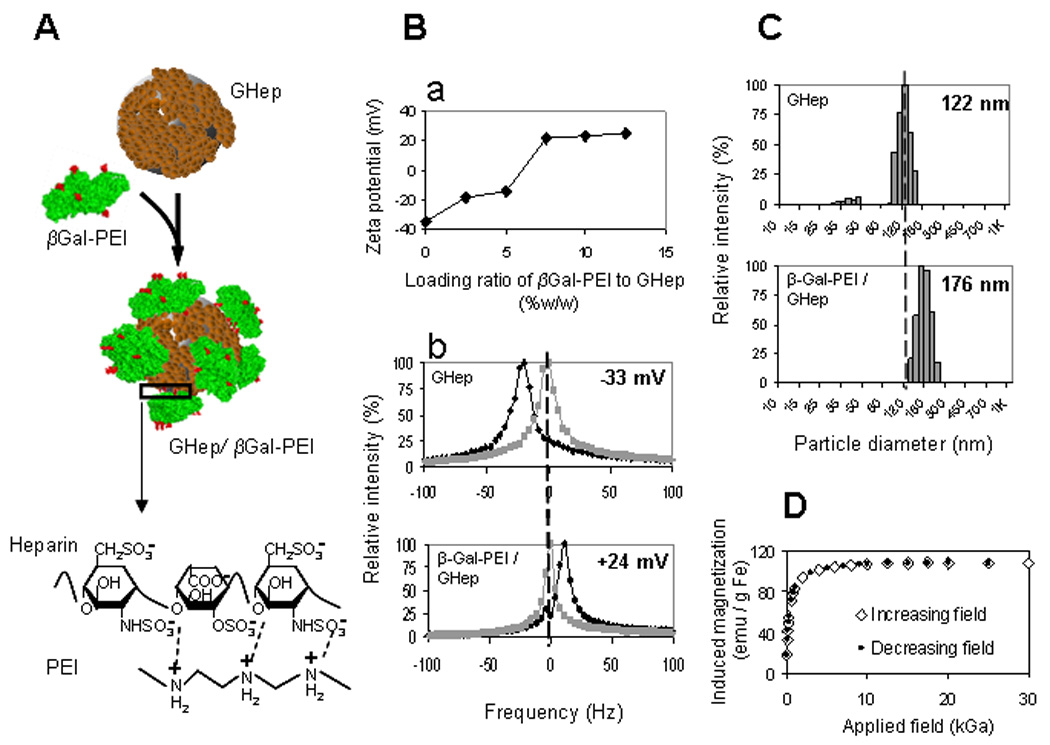
(A) PEI-modified (red) β-Galactosidase (βGal-PEI, green) is adsorbed onto the surface of heparin-coated nanoparticle (GHep, brown) to create GHep/βGal-PEI complex via electrostatic interactions between positively charged PEI and negatively charged heparin (magnified region). (Ba) ζ-potential profile as a function of βGal-PEI/GHep loading ratio exhibits saturation behavior and indicates protein loading capacity of 7.5%w/w. (Bb) Shift in ζ-potential (measured at pH=5.5) from −33 mV to +24 mV (black and gray traces represent the test and the reference measurements, acquired with and without an electric field, respectively). (C) Increase in mean particle diameter from 122 nm to 176 nm for GHep and GHep/βGal-PEI, respectively, confirm complexation. (D) Magnetization of protein-loaded nanoparticles demonstrate coinciding profiles with increasing and decreasing applied magnetic fields with no hysteresis, characteristic of superparamagnetic behavior.
